推薦產品
一般說明
Thimet oligopeptidase is considered essential in the degradation of collagen in collaboration with collagenolytic enzymes. Thimet oligopeptidase (TOP) is a neuropeptidase involved in the hydrolysis of gonadotropin-releasing hormone, a key component of the hypothalamic-pituitary-gonadal axis.
應用
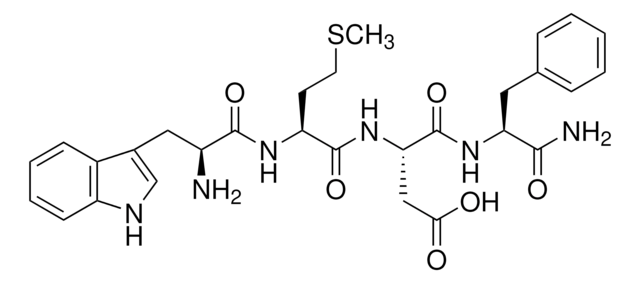
Thimet oligopeptidase can be used for the degradation of collagen in combination with collagenases. It can also be used for the hydrolysis of neuropeptides such as bradykin, neurotensin, and amyliod-β-peptide. Thimet oligopeptidase has been used in a study to investigate the effect of acute cocaine administration in male rats on TOP specific activity and mRNA levels in prosencephalic brain areas related with the reward circuitry: ventral striatum, hippocampus, and frontal cortex.
準備報告
This enzyme has been affinity chromatographically purified using a niquel affinity column. It contains a 6-Histidine tag in its C-terminus.
A working solution of this enzyme can be prepared in 20 mM phosphate buffered saline solution, pH 7.0, or sterile and deionized water, pH 7.0.
A working solution of this enzyme can be prepared in 20 mM phosphate buffered saline solution, pH 7.0, or sterile and deionized water, pH 7.0.
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Inhalation - Skin Irrit. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
T John Wu et al.
Journal of neuroendocrinology, 21(4), 293-298 (2009-02-13)
Gonadotrophin-releasing hormone (GnRH) was first isolated in the mammal and shown to be the primary regulator of the reproductive system through its initiation of pituitary gonadotrophin release. Subsequent to its discovery, this form of GnRH has been shown to be
Lilian C Russo et al.
Proteomics, 12(17), 2641-2655 (2012-06-29)
Protein interactions are crucial for most cellular process. Thus, rationally designed peptides that act as competitive assembly inhibitors of protein interactions by mimicking specific, determined structural elements have been extensively used in clinical and basic research. Recently, mammalian cells have
Lisa A Bruce et al.
The FEBS journal, 275(22), 5607-5617 (2008-10-31)
Thimet oligopeptidase (EC 3.4.24.15) is a zinc(II) endopeptidase implicated in the processing of numerous physiological peptides. Although its role in selecting and processing peptides is not fully understood, it is believed that flexible loop regions lining the substrate-binding site allow
Akio Kawasaki et al.
The Journal of biological chemistry, 285(45), 34972-34980 (2010-09-08)
Pz-peptidase A, from the thermophilic bacterium Geobacillus collagenovorans MO-1, hydrolyzes a synthetic peptide substrate, 4-phenylazobenzyloxycarbonyl-Pro-Leu-Gly-Pro-D-Arg (Pz-PLGPR), which contains a collagen-specific tripeptide sequence, -Gly-Pro-X-, but does not act on collagen proteins themselves. The mammalian enzyme, thimet oligopeptidase (TOP), which has comparable
Maurício F M Machado et al.
Biochemical and biophysical research communications, 394(2), 429-433 (2010-03-17)
Thimet oligopeptidase (EC 3.4.24.15, TOP) is a metallo-oligopeptidase that participates in the intracellular metabolism of peptides. Predictions based on structurally analogous peptidases (Dcp and ACE-2) show that TOP can present a hinge-bend movement during substrate hydrolysis, what brings some residues
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務