推薦產品
品質等級
化驗
≥98% (perchloric acid titration)
形狀
powder
溶解度
DMF: 25 mg/mL, clear, yellow-green
SMILES 字串
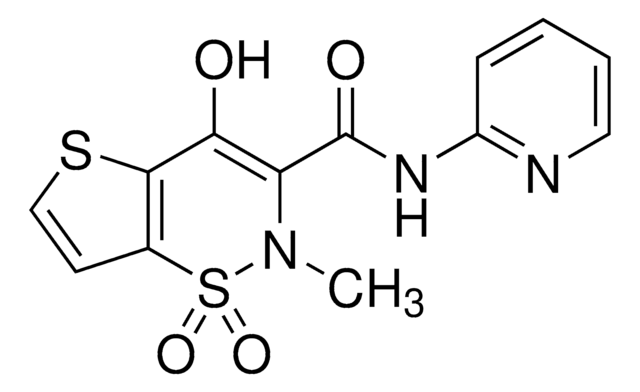
O=C(NC1=NC=CC=C1)C2=C(O)C(SC=C3)=C3S(N2C)(=O)=O
InChI
1S/C13H11N3O4S2/c1-16-10(13(18)15-9-4-2-3-6-14-9)11(17)12-8(5-7-21-12)22(16,19)20/h2-7,17H,1H3,(H,14,15,18)
InChI 密鑰
LZNWYQJJBLGYLT-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
應用
Tenoxicam has been used:
- as a non-steroidal anti-inflammatory agent (NSAID) to study its effects on root gravitropism in Arabidopsis thaliana
- as a standard in microanalysis of NSAIDs by spectrophotometry
- to test its effect on surface potential andmembrane fluidity modification in phosphoglyceride monolayers
生化/生理作用
Tenoxicam (TX) possesses antipyretic and analgesic effects. It elicits radical scavenging activity and has the potential to treat enkylosing spondylitis, extra-articular diseases, acute gout, and rheumatic diseases. It is also effective in treating primary dysmenorrhea, postpartum uterine contraction pain, and post-operation backaches. TX is capable of inhibiting prostaglandin synthesis.
Non-steroidal antiinflammatory drug (NSAID) with comparatively low risk of renal or hepatic toxicity.
訊號詞
Danger
危險分類
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 2
個人防護裝備
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
Hye Sun Gwak et al.
International journal of pharmaceutics, 236(1-2), 57-64 (2002-03-14)
The effects of vehicles and penetration enhancers on the in vitro permeation of tenoxicam from saturated solutions through dorsal hairless mouse skin were investigated. Various types of vehicles, including ester-, alcohol-, and ether-types and their mixtures, were used as vehicles
Katarzyna Czapla et al.
Langmuir : the ACS journal of surfaces and colloids, 26(5), 3485-3492 (2009-12-25)
Meloxicam, piroxicam, and tenoxicam belong to a highly potent oxicam group of nonsteroidal anti-inflammatory drugs. Whereas the structurally similar oxicams have different pharmacokinetics, treatment efficiency, and adverse effects, their common mechanism of action is the inhibition of a membrane enzyme
H O Ammar et al.
International journal of pharmaceutics, 405(1-2), 142-152 (2010-12-07)
Tenoxicam is a non steroidal anti-inflammatory drug (NSAID) widely used in the treatment of rheumatic diseases and characterized by its good efficacy and less side effects compared to other NSAIDs. Its oral administration is associated with severe side effects in
Jagdishwar R Patel et al.
Journal of pharmaceutical sciences, 101(2), 641-663 (2011-11-19)
Tenoxicam is a poorly soluble nonsteroidal anti-inflammatory drug. In this work, the solubility of tenoxicam is enhanced using amorphous spray-dried dispersions (SDDs) prepared using two molar equivalents of l-arginine and optionally with 10%-50% (w/w) polyvinylpyrrolidone (PVP). When added to the
Sutapa Mondal Roy et al.
Langmuir : the ACS journal of surfaces and colloids, 27(24), 15054-15064 (2011-10-18)
Membrane fusion is an essential process guiding many important biological events, which most commonly requires the aid of proteins and peptides as fusogenic agents. Small drug induced fusion at low drug concentration is a rare event. Only three drugs, namely
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務