SML2844
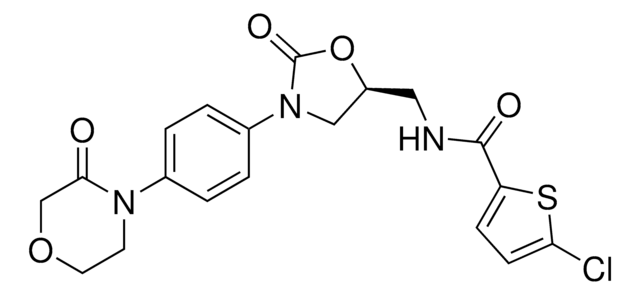
Rivaroxaban
≥98% (HPLC)
同義詞:
(S)-Rivaroxaban, (S)-5-Chloro-N-{[2-oxo-3-[4-(3-oxomorpholin-4-yl)phenyl]oxazolidin-5-yl]methyl} thiophene-2-carboxamide, 5-Chloro-N-({(5S)-2-oxo-3-[4-(3-oxomorpholin-4-yl)phenyl]-1,3-oxazolidin-5-yl}methyl)thiophene-2-carboxamide, BAY 59-7939, BAY-59-7939, BAY59-7939, 5-Chloro-N-[[(5S)-2-oxo-3-[4-(3-oxo-4-morpholinyl)phenyl]-5-oxazolidinyl]methyl]-2-thiophenecarboxamide
About This Item
推薦產品
品質等級
化驗
≥98% (HPLC)
形狀
powder
光學活性
[α]/D -34 to -44, c = 0.3 in DMSO
顏色
white to beige
溶解度
DMSO: 2 mg/mL, clear
儲存溫度
2-8°C
SMILES 字串
[s]1c(ccc1C(=O)NC[C@@H]2OC(=O)N(C2)c3ccc(cc3)N4CCOCC4=O)Cl
InChI
1S/C19H18ClN3O5S/c20-16-6-5-15(29-16)18(25)21-9-14-10-23(19(26)28-14)13-3-1-12(2-4-13)22-7-8-27-11-17(22)24/h1-6,14H,7-11H2,(H,21,25)/t14-/m0/s1
InChI 密鑰
KGFYHTZWPPHNLQ-AWEZNQCLSA-N
尋找類似的產品? 前往 產品比較指南
生化/生理作用
危險聲明
危險分類
Aquatic Chronic 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務