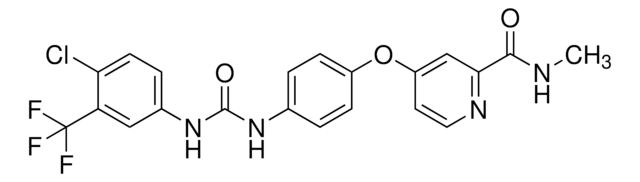
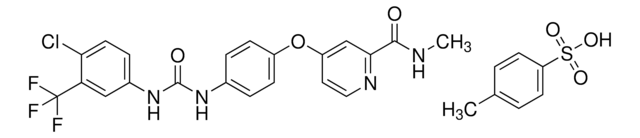
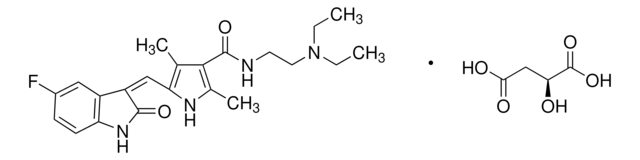
SML2633
Sorafenib tosylate
≥98% (HPLC)
同義詞:
4-[4-[[[[4-Chloro-3-(trifluoromethyl)phenyl]amino]carbonyl]amino]phenoxy]-N-methyl-2-pyridinecarboxamide, 4-methylbenzenesulfonate, BAY 43-9006 tosylate salt, BAY43-9006 tosylate salt, N-[4-Chloro-3-(trifluoromethyl)phenyl]-N′-[4-[2-(N-methylcarbamoyl)-4-pyridyloxy]phenyl]urea, 4-methylbenzenesulfonate
About This Item
推薦產品
化驗
≥98% (HPLC)
形狀
powder
儲存條件
desiccated
顏色
white to very dark brown
溶解度
DMSO: 2 mg/mL, clear
儲存溫度
2-8°C
InChI
1S/C21H16ClF3N4O3.C7H8O3S/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25;1-6-2-4-7(5-3-6)11(8,9)10/h2-11H,1H3,(H,26,30)(H2,28,29,31);2-5H,1H3,(H,8,9,10)
InChI 密鑰
IVDHYUQIDRJSTI-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
生化/生理作用
訊號詞
Danger
危險分類
Aquatic Acute 1 - Aquatic Chronic 1 - Lact. - Repr. 1B - STOT RE 1
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務