推薦產品
生物源
(from Biopolaris leersia fungal metabolite)
品質等級
化驗
≥95% (HPLC)
形狀
powder
儲存條件
protect from light
顏色
white to beige
儲存溫度
−20°C
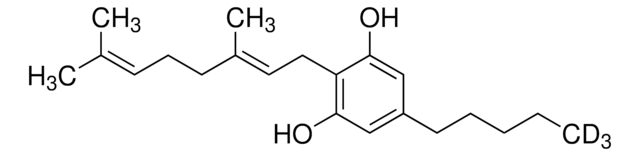
SMILES 字串
[H]C(/C1=C/C[C@]([C@]2(O[C@@H](C=C(C)C)C[C@@H]2C)CC3)([H])[C@@]3(C)C[C@@]([C@](C)(O)C4)([H])[C@]1([H])C4=O)=O
InChI
1S/C25H36O4/c1-15(2)10-18-11-16(3)25(29-18)9-8-23(4)12-19-22(20(27)13-24(19,5)28)17(14-26)6-7-21(23)25/h6,10,14,16,18-19,21-22,28H,7-9,11-13H2,1-5H3/b17-6-/t16-,18-,19-,21+,22+,23+,24+,25-/m0/s1
InChI 密鑰
MWYYLZRWWNBROW-BDZRSQQBSA-N
生化/生理作用
Ophiobolin A is a fungal metabolite, toxic to many crops and found to have antimicrobial and anticancer activity. Ophiobolin A is a calmodulin antagonist and inhibitor of big conductance Ca2+-activated K+ channel (BKCa) activity. Its anticancer activity may involve inhibition of multiple oncogenic signaling pathways including PI3K/mTOR, Ras/Raf/ERK and CDK/RB. One study suggested the possibility of pyrrolylation of lysine residues on its intracellular target protein(s) as a mechanism of action on various targets.
Ophiobolin A is a fungal metabolite; calmodulin antagonist with anticancer activity.
其他說明
Light and air sensitive.
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務