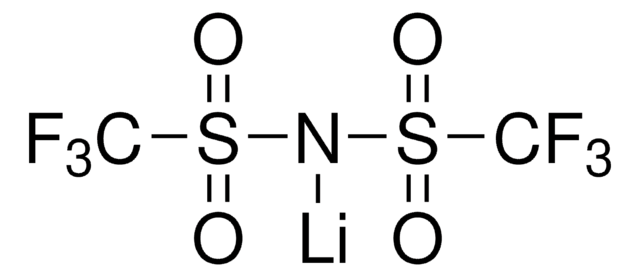
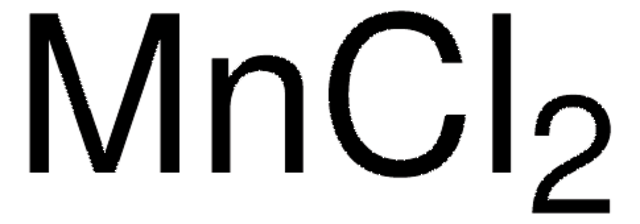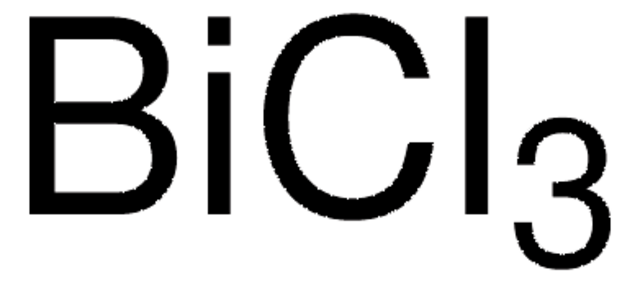推薦產品
品質等級
化驗
≥98% (HPLC)
形狀
powder
顏色
white to light brown
溶解度
DMSO: 1 mg/mL, clear (warmed)
儲存溫度
−20°C
SMILES 字串
O=C(CN1CCN(CC)CC1)NC2=CC=C(C3=CC=CC4=C3OC(N5CCOCC5)=CC4=O)C6=C2C(C=CC=C7)=C7S6
InChI
1S/C33H34N4O4S/c1-2-35-12-14-36(15-13-35)21-29(39)34-26-11-10-23(33-31(26)25-6-3-4-9-28(25)42-33)22-7-5-8-24-27(38)20-30(41-32(22)24)37-16-18-40-19-17-37/h3-11,20H,2,12-19,21H2,1H3,(H,34,39)
InChI 密鑰
AATCBLYHOUOCTO-UHFFFAOYSA-N
生化/生理作用
KU-0060648 is a very potent dual inhibitor of DNA-PK and PI3K. The IC50 values for inhibition of DNA-PK in MCF7 and SW620 cells are 19 and 170 nM, respectively. The compound KU-0060648 inhibits MCF7 PI3K activity with an IC50 of 39 nM, but is inactive against PI3K in SW620, suggesting a cell-dependent mechanism of inhibition. KU-0060648 increases sensitivity to DNA damaging cytotoxic drugs such as etoposide and doxorubicin in tumor cell lines expressing DNA-PK. KU-0060648 stimulates Cas9 (CRISPR associated protein 9) mediated genome editing by enhancing the rate of homology directed-repair (HDR) and decreasing the rate of NHEJ (non-homologous end-joining).
KU-0060648 is observed to inhibit proliferation and initiate apoptosis in hepatocellular carcinoma. KU-0060648 is useful in chemotherapy as it increases the efficiency of double stranded breaks initiated by anticancer drugs.
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Joanne M Munck et al.
Molecular cancer therapeutics, 11(8), 1789-1798 (2012-05-12)
DNA double-strand breaks (DSB) are the most cytotoxic lesions induced by topoisomerase II poisons. Nonhomologous end joining (NHEJ) is a major pathway for DSB repair and requires DNA-dependent protein kinase (DNA-PK) activity. DNA-PK catalytic subunit (DNA-PKcs) is structurally similar to
KU-0060648 inhibits hepatocellular carcinoma cells through DNA-PKcs-dependent and DNA-PKcs-independent mechanisms.
Chen M B, et al.
Oncotarget, 7(13), 17047-17047 (2016)
Developments of DNA-dependent protein kinase inhibitors as anticancer agents.
Ma C C, et al.
Mini-Reviews in Medicinal Chemistry, 14(11), 884-895 (2014)
Valentina Perini et al.
International journal of molecular sciences, 21(21) (2020-11-11)
Poly(ADP-ribosyl)polymerase (PARP) synthesizes poly(ADP-ribose) (PAR), which is anchored to proteins. PAR facilitates multiprotein complexes' assembly. Nuclear PAR affects chromatin's structure and functions, including transcriptional regulation. In response to stress, particularly genotoxic stress, PARP activation facilitates DNA damage repair. The PARP
Francis Robert et al.
Genome medicine, 7, 93-93 (2015-08-27)
The ability to modify the genome of any cell at a precise location has drastically improved with the recent discovery and implementation of CRISPR/Cas9 editing technology. However, the capacity to introduce specific directed changes at given loci is hampered by
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務