推薦產品
品質等級
化驗
≥98% (HPLC)
形狀
powder
儲存條件
desiccated
顏色
white to beige
溶解度
DMSO: >2 mg/mL (warmed)
儲存溫度
2-8°C
SMILES 字串
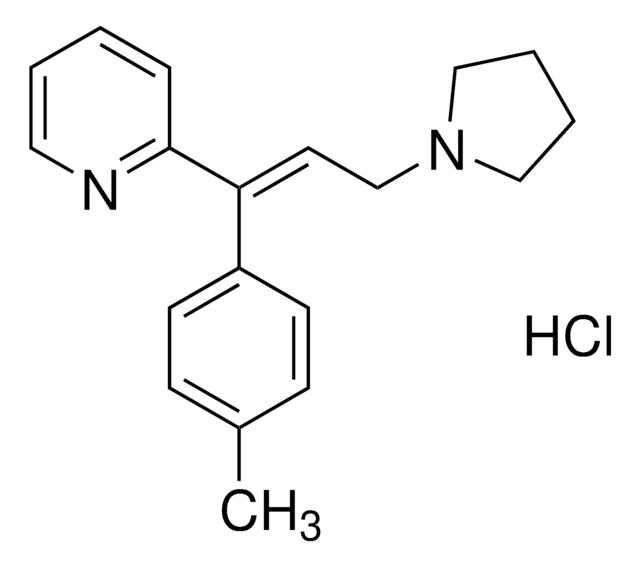
Cc1ccc(cc1)\C(=C/CN2CCCC2)c3cccc(\C=C\C(O)=O)n3
InChI
1S/C22H24N2O2/c1-17-7-9-18(10-8-17)20(13-16-24-14-2-3-15-24)21-6-4-5-19(23-21)11-12-22(25)26/h4-13H,2-3,14-16H2,1H3,(H,25,26)/b12-11+,20-13+
InChI 密鑰
PWACSDKDOHSSQD-IUTFFREVSA-N
基因資訊
human ... HRH1(3269)
應用
Acrivastine has been used as an antihistamine to investigate the relation between the increased residence time of antihistamine at the histamine H1 receptor (H1R) and the duration of effective target-inhibition by this antagonist.
生化/生理作用
Acrivastine is a second-generation H1-receptor antagonist.
Acrivastine is a second-generation antihistamine, an H1-receptor antagonist.
Acrivastine is a second-generation antihistamine. It is a derivative of first-generation compound triprolidine. Acrivastine is effectively used for treating allergic diseases including cholinergic urticaria and histamine-medicated dermatoses.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
R D Mann et al.
BMJ (Clinical research ed.), 320(7243), 1184-1186 (2000-04-28)
To investigate the frequency with which sedation was reported in post-marketing surveillance studies of four second generation antihistamines: loratadine, cetirizine, fexofenadine, and acrivastine. Prescription-event monitoring studies. Prescriptions were obtained for each cohort in the immediate post-marketing period. Event data were
Xiaochen Gu et al.
Journal of pharmaceutical and biomedical analysis, 37(4), 663-667 (2005-03-31)
High-performance liquid chromatography (HPLC) was used for the simultaneous quantification of the H(1)-antihistamine acrivastine and the decongestant pseudoephedrine hydrochloride. Both compounds were detected at the wavelength of 214 nm. The influence of the mobile phase and the detection wavelength was
M J Mattila et al.
European journal of clinical pharmacology, 55(2), 85-93 (1999-05-21)
Most of the modern non-sedating H1 receptor antagonists (antihistamines) penetrate the brain poorly, allowing the use of doses large enough to counteract allergic processes in peripheral tissues without important central effects. The antihistamines reviewed here are acrivastine, astemizole, cetirizine, ebastine
A Reimers et al.
Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology, 32(12), 1763-1768 (2003-03-26)
Leukotriene receptor antagonists have shown some efficacy in t he treatment of asthma. Injection of LTC4, LTD4 and LTE4 into the skin leads to a weal-and-flare reaction, suggesting an involvement of leukotrienes in the pathogenesis of urticaria. Indeed, various reports
Róbert Kiss et al.
European journal of medicinal chemistry, 39(11), 959-967 (2004-10-27)
Three-dimensional model of the human histamine H1 receptor was developed by homology modelling using the high resolution structure of bovine rhodopsin as template. Genetic algorithm based docking calculations were used to identify the role of several amino acids having an
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務