SML0015
SR 142948A
≥98% (HPLC)
同義詞:
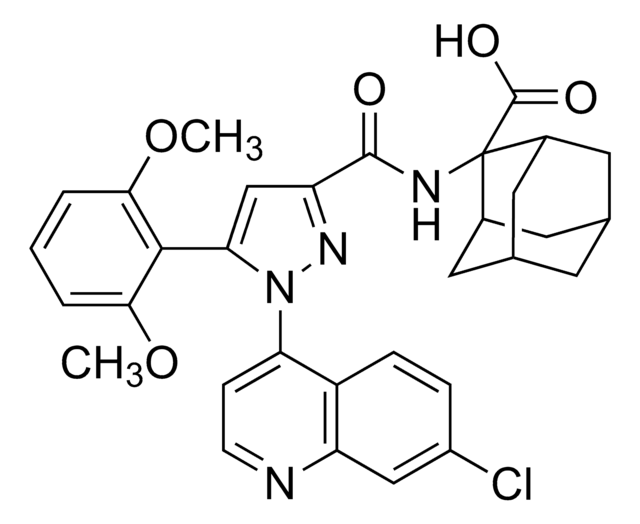
2-[[5-(2,6-dimethoxyphenyl)-1-(4-(N-(3-dimethylaminopropyl)-N-methylcarbamoyl)-2-isopropylphenyl)-1H-pyrazole3-carbonyl]amino] adamantane-2-carboxylic acid hydrochloride, SR 142948 hydrochloride
About This Item
推薦產品
化驗
≥98% (HPLC)
形狀
powder
顏色
white to beige
溶解度
H2O: ≥2 mg/mL at warmed to 60 °C
起源
Sanofi Aventis
儲存溫度
2-8°C
SMILES 字串
Cl.COc1cccc(OC)c1-c2cc(nn2-c3ccc(cc3C(C)C)C(=O)N(C)CCCN(C)C)C(=O)NC4([C@@H]5C[C@H]6C[C@@H](C5)C[C@@H]4C6)C(O)=O
InChI
1S/C39H51N5O6.ClH/c1-23(2)29-21-26(37(46)43(5)15-9-14-42(3)4)12-13-31(29)44-32(35-33(49-6)10-8-11-34(35)50-7)22-30(41-44)36(45)40-39(38(47)48)27-17-24-16-25(19-27)20-28(39)18-24;/h8,10-13,21-25,27-28H,9,14-20H2,1-7H3,(H,40,45)(H,47,48);1H/t24-,25+,27-,28+,39?;
InChI 密鑰
CUYNEHGBVHUQQW-BVJGGMLGSA-N
應用
生化/生理作用
特點和優勢
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務