推薦產品
生物源
mouse
抗體表格
purified from hybridoma cell culture
抗體產品種類
primary antibodies
無性繁殖
COL-10, monoclonal
形狀
buffered aqueous solution
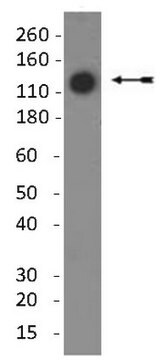
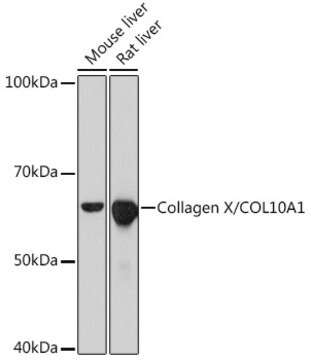
分子量
~60 kDa
物種活性
deer, porcine, human
包裝
antibody small pack of 25 μL
濃度
~1 mg/mL
技術
immunoblotting: suitable
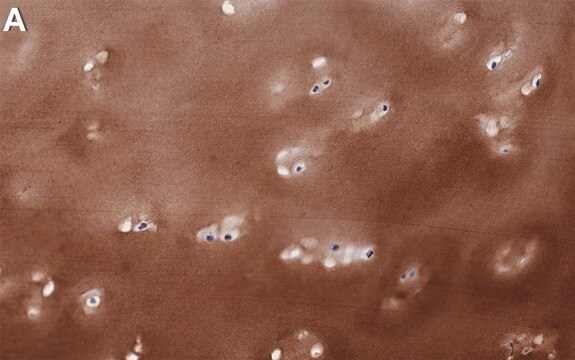
immunofluorescence: 5-10 μg/mL using human osteosarcoma SaOS-2 cells
immunohistochemistry: suitable
同型
IgM
UniProt登錄號
運輸包裝
dry ice
儲存溫度
−20°C
目標翻譯後修改
unmodified
基因資訊
human ... COL10A1(1300)
一般說明
The extracellular matrix (ECM) found in the extracellular environment of all tissues and organs, provides the physical microenvironment for cells and a substrate for cell anchorage. It serves as a tissue scaffold and is a dynamic structure whose organization and composition modulate various cellular processes including cell proliferation, attachment, migration, differentiation and survival. The composition of the extracellular framework of all vertebrates is dominated by a Collagen protein family, each member with unique features suited for its function and location.
Type X collagen,also known as Collagen alpha-1(X) chain (COL10A1), is a product of hypertrophic chondrocytes. It shares a similar domain structure with type VIII collagen. In addition, both collagen types represent major components of hexagonal lattice structure, in which the collagen molecules link together by interactions involving the non-triple-helical end regions. Despite these similarities, a distinct tissue distribution has been found for these two molecules: type VIII collagen is distributed in various tissues, whereas type X is restricted to normal fetal hypertrophic cartilage in the growth zones of long bones, vertebrae and ribs and in adult (> 21 yr) thyroid cartilage. It is also found in bone fracture callus, osteoarthritic cartilage and chondrogenic neoplasms, and may be involved in cartilage mineralization. Type X collagen is non-fibrillar, but forms fine pericellular filaments in association with cartilage collagen. It interacts with matrix proteins, such as connexin V, chondrocalcein, collagen II and proteoglycans, as well as with Ca2+ . Mutations in this gene are associated with schmid metaphyseal chondroplasia (MCDS).
The development of antibodies against collagens has provided a powerful method for examining the distribution of these connective tissue proteins and for investigation of epithelial-mesenchymal interactions, tumorigenesis and basement membrane biology in ontogeny and epithelial differentiation.8 Antibodies that react specifically with collagen type X are useful for the study of specific differential tissue expression and the localization of collagen type X.
Type X collagen,also known as Collagen alpha-1(X) chain (COL10A1), is a product of hypertrophic chondrocytes. It shares a similar domain structure with type VIII collagen. In addition, both collagen types represent major components of hexagonal lattice structure, in which the collagen molecules link together by interactions involving the non-triple-helical end regions. Despite these similarities, a distinct tissue distribution has been found for these two molecules: type VIII collagen is distributed in various tissues, whereas type X is restricted to normal fetal hypertrophic cartilage in the growth zones of long bones, vertebrae and ribs and in adult (> 21 yr) thyroid cartilage. It is also found in bone fracture callus, osteoarthritic cartilage and chondrogenic neoplasms, and may be involved in cartilage mineralization. Type X collagen is non-fibrillar, but forms fine pericellular filaments in association with cartilage collagen. It interacts with matrix proteins, such as connexin V, chondrocalcein, collagen II and proteoglycans, as well as with Ca2+ . Mutations in this gene are associated with schmid metaphyseal chondroplasia (MCDS).
The development of antibodies against collagens has provided a powerful method for examining the distribution of these connective tissue proteins and for investigation of epithelial-mesenchymal interactions, tumorigenesis and basement membrane biology in ontogeny and epithelial differentiation.8 Antibodies that react specifically with collagen type X are useful for the study of specific differential tissue expression and the localization of collagen type X.
免疫原
Porcine collagen type X
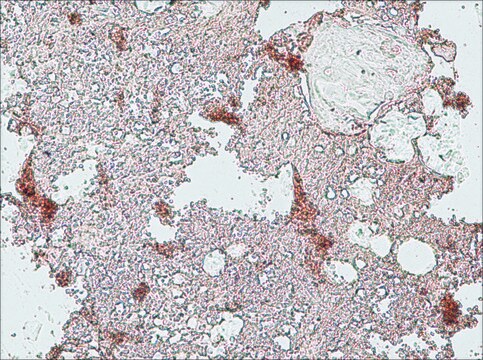
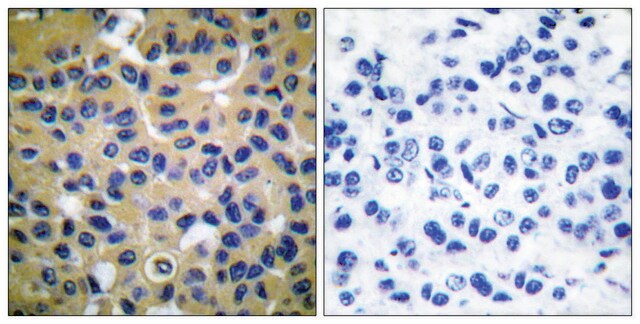
應用
Monoclonal Anti-Collagen, Type X specifically recognizes native and denatured collagen type X. It does not recognize collagen types I, II, III, V, IX and XI. Reactivity has been observed with human, deer and porcine collagen type X. The antibody is recommended to use in various immunological techniques, including Immunoblotting (∼ 60 kDa in denatured-reduced preparation), Immunofluorescence and Immunohistochemistry.
外觀
Supplied as a solution in 0.01 M phosphate buffered saline pH 7.4, containing 15 mM sodium azide as a preservative.
其他說明
This product is for R&D use only, not for drug, household, or other uses.
未找到適合的產品?
試用我們的產品選擇工具.
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
T Aigner et al.
Histochemistry and cell biology, 107(6), 435-440 (1997-06-01)
Little is known about matrix biochemistry and cell differentiation patterns in chondrogenic neoplasms. This is the first description of the focal expression of collagen type X by neoplastic chondrocytes in situ and its incorporation into the extracellular matrix of cartilaginous
G J Rucklidge et al.
Matrix biology : journal of the International Society for Matrix Biology, 15(2), 73-80 (1996-07-01)
Intact collagen type X cannot readily be extracted from the growth plate. Both the use of pepsin to release this molecule from tissue and the relative solubility of collagen type X following treatment of chick embryos with beta-aminopropionitrile (Chen et
I Girkontaite et al.
Matrix biology : journal of the International Society for Matrix Biology, 15(4), 231-238 (1996-09-01)
For studies on processing and tissue distribution of type X collagen, monoclonal antibodies were prepared against human recombinant collagen type X (hrCol X) and tested by ELISA, immunoblotting and immunohistology. Forty-two clones were obtained which were grouped into four different
N Yamaguchi et al.
The Journal of biological chemistry, 264(27), 16022-16029 (1989-09-25)
We have isolated two overlapping cDNA clones covering 2425 base pairs encoding a short type VIII collagen chain synthesized by rabbit corneal endothelial cells. The cDNAs encode an open reading frame of 744 amino acid residues containing a triple-helical domain
Hypertrophic cartilage matrix. Type X collagen, supramolecular assembly, and calcification.
T M Schmid et al.
Annals of the New York Academy of Sciences, 580, 64-73 (1990-01-01)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務