推薦產品
品質等級
化驗
≥98% (HPLC)
溶解度
DMSO: >20 mg/mL
儲存溫度
2-8°C
SMILES 字串
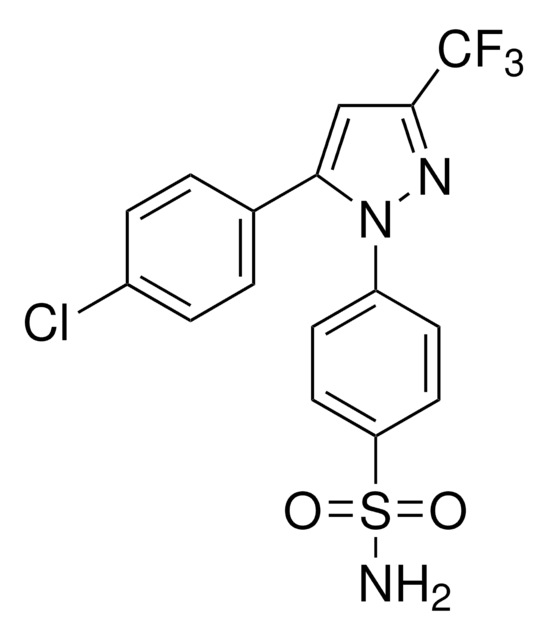
COc1ccc(cc1)-n2nc(cc2-c3ccc(Cl)cc3)C(F)(F)F
InChI
1S/C17H12ClF3N2O/c1-24-14-8-6-13(7-9-14)23-15(10-16(22-23)17(19,20)21)11-2-4-12(18)5-3-11/h2-10H,1H3
InChI 密鑰
PQUGCKBLVKJMNT-UHFFFAOYSA-N
基因資訊
human ... PTGS1(5742) , PTGS2(5743)
應用
SC-560 has been used as a cyclooxygenase-1 (COX-1) inhibitor to study its effects on prostaglandin E-2 (PGE2) signaling in ciliogenesis in zebrafish embryos. It has also been used as a selective inhibitor of COX-1 to study its role in PM10-induced endothelial dysfunction.
生化/生理作用
SC-560 (5-(4-chlorophenyl)-1-(4-methoxyphenyl)-3-(trifluoromethyl)-1H-pyrazole) is a non-steroidal anti-inflammatory drug (NSAID). It is a lipophilic, diaryl heterocyclic compound. SC-560 acts as an effective antiviral agent against hepatitis C virus (HCV). It also has a potential to hinder prostaglandin E2 synthesis in neurons at nanomolar concentrations.
SC-560 belongs to the diaryl heterocycle class of cyclooxygenase (COX) inhibitors. It exhibits anti-tumor and anti-proliferative activities.
Selective cyclooxygenase-1 (COX-1) inhibitor, exhibiting 700-fold selectivity for COX-1 over COX-2.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Formulation dependent pharmacokinetics, bioavailability and renal toxicity of a selective cyclooxygenase-1 inhibitor SC-560 in the rat
Teng XW, et al.
Toxicology and Applied Pharmacology, 272(1), 205-210 (2003)
Alessandra Pannunzio et al.
Pharmaceuticals (Basel, Switzerland), 11(4) (2018-10-14)
Prostaglandins and thromboxane are lipid signaling molecules deriving from arachidonic acid by the action of the cyclooxygenase isoenzymes COX-1 and COX-2. The role of cyclooxygenases (particularly COX-2) and prostaglandins (particularly PGE₂) in cancer-related inflammation has been extensively investigated. In contrast
Inhibition of prostaglandin E2 synthesis by SC-560 is independent of cyclooxygenase 1 inhibition
Brenneis C, et al.
Faseb Journal, 20(9), 1352-1360 (2006)
Cyclooxygenase-1 (COX-1) and COX-1 inhibitors in cancer: a review of oncology and medicinal chemistry literature
Pannunzio A and Coluccia M
Pharmaceuticals (Basel, Switzerland), 11(4), 101-101 (2018)
Synthesis of Celecoxib, Mavacoxib, SC-560, Fluxapyroxad, and Bixafen Enabled by Continuous Flow Reaction Modules
Britton J, et al.
European Journal of Organic Chemistry, 2017(44), 6566-6574 (2017)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務