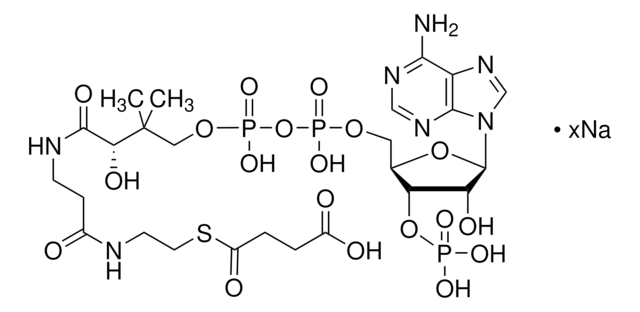
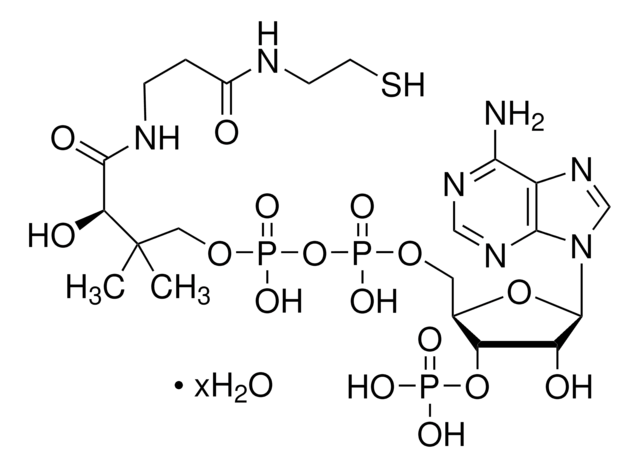
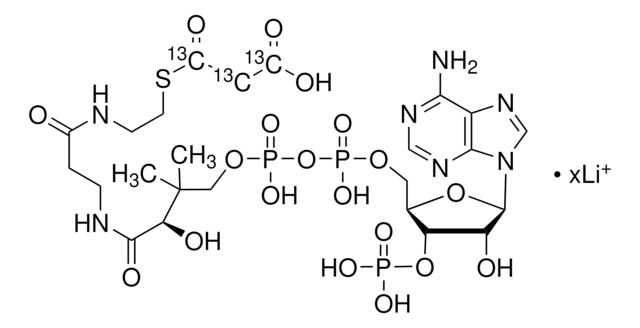
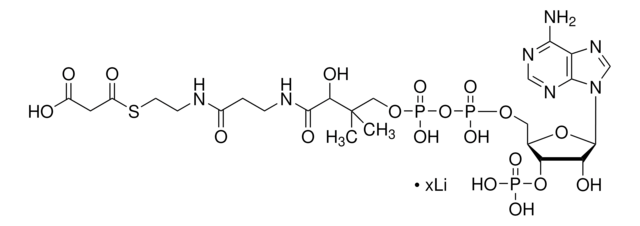
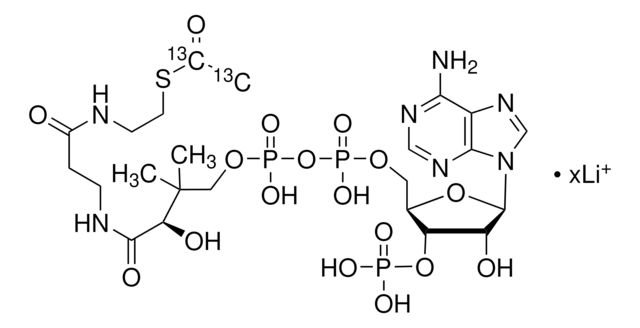
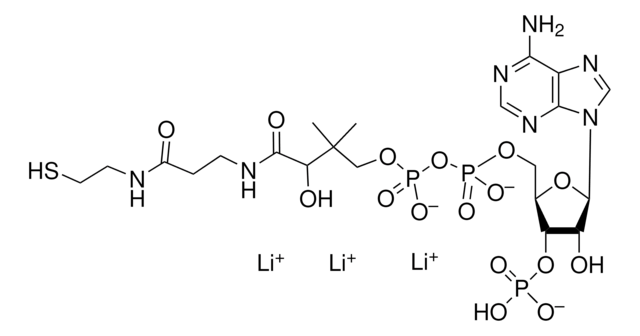
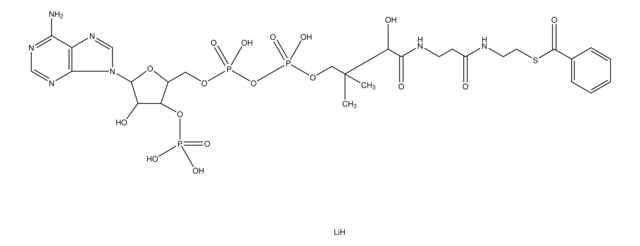
About This Item
推薦產品
品質等級
化驗
≥90% (HPLC)
溶解度
H2O: soluble 50 mg/mL protein, clear, colorless
儲存溫度
−20°C
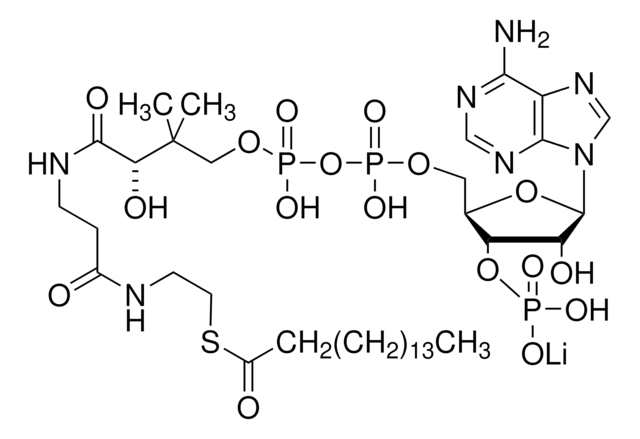
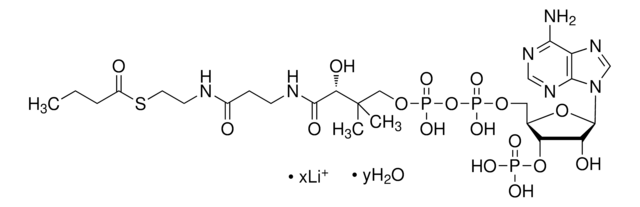
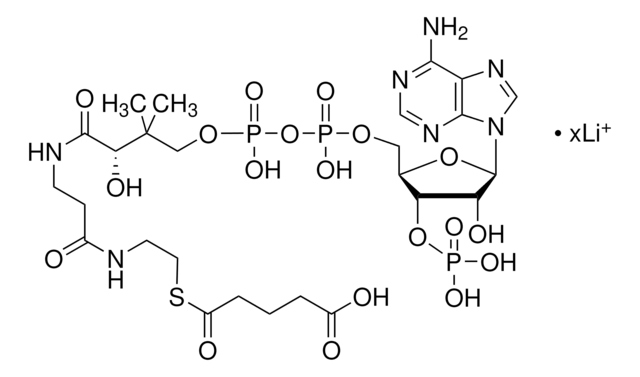
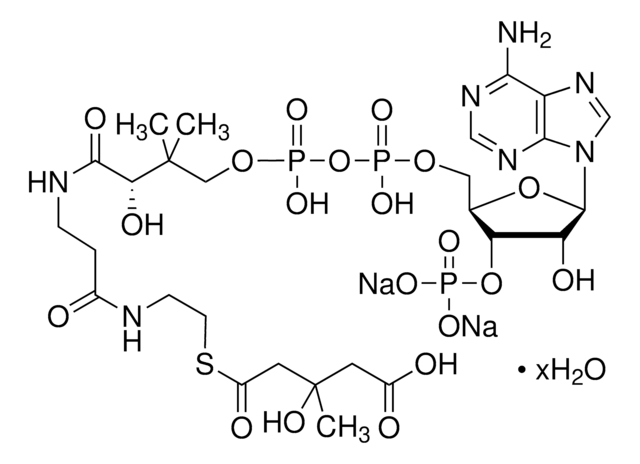
SMILES 字串
[Li].CC(C)(COP(O)(=O)OP(O)(=O)OCC1OC(C(O)C1OP(O)(O)=O)n2cnc3c(N)ncnc23)C(O)C(=O)NCCC(=O)NCCSC(=O)CC(O)=O
InChI
1S/C24H38N7O19P3S.Li/c1-24(2,19(37)22(38)27-4-3-13(32)26-5-6-54-15(35)7-14(33)34)9-47-53(44,45)50-52(42,43)46-8-12-18(49-51(39,40)41)17(36)23(48-12)31-11-30-16-20(25)28-10-29-21(16)31;/h10-12,17-19,23,36-37H,3-9H2,1-2H3,(H,26,32)(H,27,38)(H,33,34)(H,42,43)(H,44,45)(H2,25,28,29)(H2,39,40,41);
InChI 密鑰
OPIJLICRFQMMJH-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
應用
- Krebs Ringer碳酸氢盐培养基,用于肪酸氧化测定之中的胰蛋白酶酶解和重悬成纤维细胞预培养
- 脂肪酸合酶闪烁邻近测定的HEPES(4-(2-羟乙基)-1-哌嗪乙磺酸)缓冲液
- 用作琥珀酰辅酶A连接酶测定中的反应混合物内标
生化/生理作用
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
客戶也查看了
文章
Information on fatty acid synthesis and metabolism in cancer cells. Learn how proliferatively active cells require fatty acids for functions such as membrane generation, protein modification, and bioenergetic requirements. These fatty acids are derived either from dietary sources or are synthesized by the cell.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務