全部照片(1)
About This Item
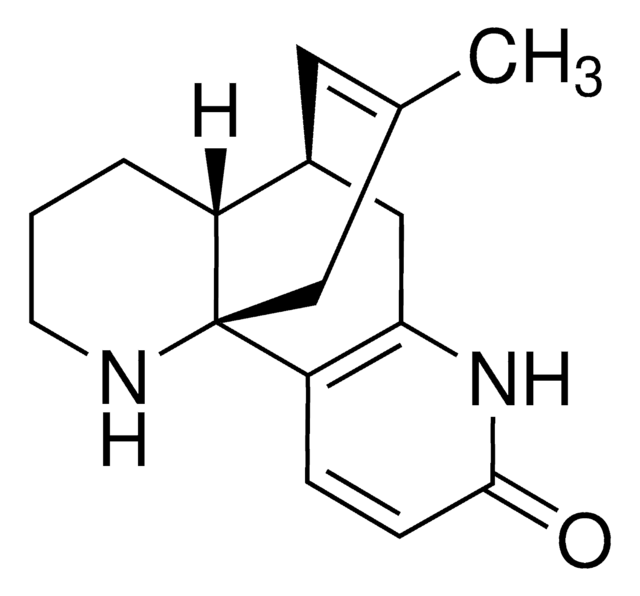
經驗公式(希爾表示法):
C15H18N2O
CAS號碼:
分子量::
242.32
MDL號碼:
分類程式碼代碼:
12352200
PubChem物質ID:
NACRES:
NA.77
推薦產品
生物源
synthetic
品質等級
化驗
≥98% (TLC)
形狀
powder
溶解度
methanol: 10 mg/mL, clear, colorless
儲存溫度
2-8°C
SMILES 字串
C\C=C1/C2CC3=C(C=CC(=O)N3)C1(N)CC(C)=C2
InChI
1S/C15H18N2O/c1-3-11-10-6-9(2)8-15(11,16)12-4-5-14(18)17-13(12)7-10/h3-6,10H,7-8,16H2,1-2H3,(H,17,18)/b11-3+
InChI 密鑰
ZRJBHWIHUMBLCN-QDEBKDIKSA-N
基因資訊
human ... BCHE(590)
rat ... Ache(83817)
一般說明
Huperzine A is an alkaloid derived from Chinese club moss Huperzia serrata.
應用
(±)-Huperzine A has been used in anti-acetylcholinesterase bioautographic assay.
生化/生理作用
Huperzine A is an effective acetylcholinesterase inhibitor and can cross the blood-brain barrier. Huperzine A is known to prevent cognitive defects, glutamate-induced nerve cell death.It is recognized for its use in the treatment of Alzheimer′s disease and organophosphate poisoning.
抗化學/物理腐蝕性
Acetylcholinesterase inhibitor.
訊號詞
Danger
危險分類
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Women's Health in Complementary and Integrative Medicine, 138-138 (2004)
Effect of huperzine A, a new cholinesterase inhibitor, on the central cholinergic system of the rat.
X C Tang et al.
Journal of neuroscience research, 24(2), 276-285 (1989-10-01)
The present study represents the first comprehensive investigation of the effect of huperzine A (HUP-A), a new cholinesterase inhibitor (ChEI) isolated from a Lycopodium species, upon acetylcholinesterase (AChE) activity, acetylcholine (ACh) levels and release, and cholinergic receptors in rat brain
Yun Zhou et al.
Planta medica, 75(6), 568-574 (2009-02-14)
Three new saponins, bacopasides IX-XI (1- 3), together with their known analogues bacopaside I (4), bacopaside II (5), bacopasaponsin C (6), and bacopasaponsin D (7), were isolated from the whole plant of Bacopa monniera. Compounds 3, 4, and 6 showed
Coumarins from Peucedanum ostruthium. as Inhibitors of Acetylcholinesterase
Urbain A, et al.
Pharmaceutical biology, 43(8), 647-650 (2005)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務