推薦產品
product name
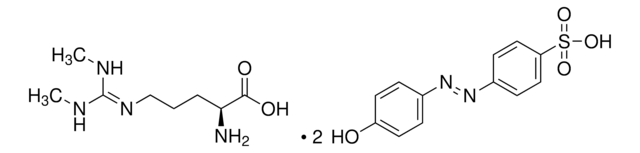
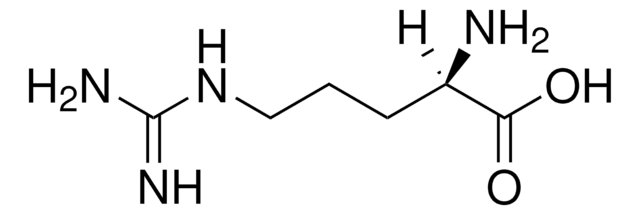
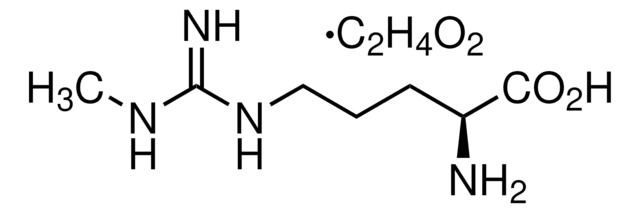
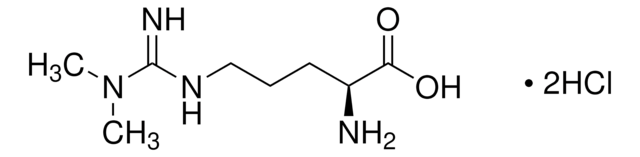
L-高精氨酸 盐酸盐, unnatural arginine analog
化驗
≥98% (TLC)
形狀
powder
顏色
white to off-white
mp
213-215 °C (lit.)
溶解度
H2O: soluble
應用
peptide synthesis
SMILES 字串
Cl[H].N[C@@H](CCCCNC(N)=N)C(O)=O
InChI
1S/C7H16N4O2.ClH/c8-5(6(12)13)3-1-2-4-11-7(9)10;/h5H,1-4,8H2,(H,12,13)(H4,9,10,11);1H/t5-;/m0./s1
InChI 密鑰
YMKBVNVCKUYUDM-JEDNCBNOSA-N
尋找類似的產品? 前往 產品比較指南
相關類別
應用
L-高精氨酸可用于产生非天然蛋白质,用于翻译后蛋白质修饰的研究。精氨酸残基常位于蛋白质和酶的活性中心。将这些精氨酸替换为高精氨酸有助于阐明蛋白质的功能和结构要求。L-高精氨酸用于研究细胞通过一氧化氮合酶 (s) 产生一氧化氮的机制。L-高精氨酸用作选择性哺乳动物碱性磷酸酶同工酶抑制剂。高精氨酸取代精氨酸或赖氨酸使蛋白质对胰蛋白酶水解产生抗性。
高精氨酸取代精氨酸或赖氨酸使蛋白质对胰蛋白酶的蛋白水解具有抗性。精氨酸残基常位于蛋白质和酶的活性中心。将这些精氨酸替换为高精氨酸有助于阐明蛋白质的功能和结构要求。
生化/生理作用
高精氨酸是组织非特异性碱性磷酸酶 (TNALP) 的抑制剂。也是通过钠非依赖性高亲和力 y + 转运体进行精氨酸细胞转运的抑制剂。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
客戶也查看了
Roman N Rodionov et al.
Journal of the American Heart Association, 8(14), e012486-e012486 (2019-07-16)
Background Homoarginine ( hA rg) has been shown to be cardioprotective in a model of ischemic heart failure; however, the mechanism remains unknown. hA rg can inhibit tissue-nonspecific alkaline phosphatase ( TNAP ), an enzyme that promotes vascular calcification. We
J Fernandes et al.
Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas, 41(1), 41-46 (2007-12-22)
Our objective was to characterize the modulation of the activity of Saccharomyces cerevisiae alkaline phosphatases (ALPs) by classic inhibitors of ALP activity, cholesterol and steroid hormones, in order to identify catalytic similarities between yeast and mammalian ALPs. S. cerevisiae expresses
Gabor Tajti et al.
Allergy, asthma, and clinical immunology : official journal of the Canadian Society of Allergy and Clinical Immunology, 14, 2-2 (2018-01-09)
Contribution of nitric-oxide (NO) pathway to the pathogenesis of bronchial asthma (asthma) is ambiguous as NO may confer both protective and detrimental effects depending on the NO synthase (NOS) isoforms, tissue compartments and underlying pathological conditions (e.g. systemic inflammation). Asymmetric
Robert N. Atkinson et al.
The Journal of organic chemistry, 64(10), 3467-3475 (2001-10-25)
Using the catalytic asymmetric Sharpless carbamate aminohydroxylation, conformationally restricted L-arginine and L-homoarginine derivatives (5-8) were prepared in good enantiomeric excess to investigate the binding requirements of L-arginine-based compounds with nitric oxide synthase. The L-arginine derivatives (5 and 6) inhibited both
Akiya Akahoshi et al.
Biochemical and biophysical research communications, 414(3), 625-630 (2011-10-18)
Arginine analogs were incorporated site-specifically into proteins using an in vitro translation system. In this system, mRNAs containing a CGGG codon were translated by an aminoacyl-tRNA(CCCG), which was charged with arginine analogs using yeast arginyl-tRNA synthetase. N(G)-monomethyl-L-arginine, L-citrulline and L-homoarginine
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務