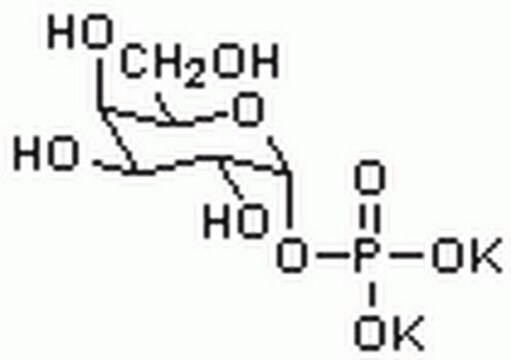
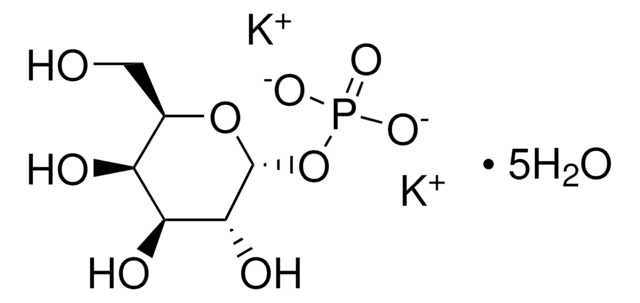
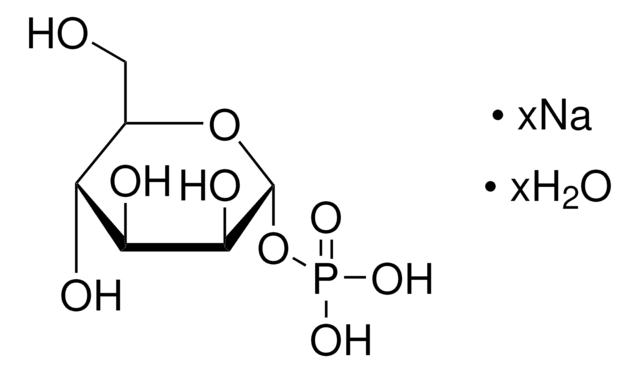
推薦產品
品質等級
化驗
≥98%
形狀
powder
技術
thin layer chromatography (TLC): suitable
溶解度
H2O: soluble 50 mg/mL, clear, colorless
儲存溫度
−20°C
SMILES 字串
NC1CCCCC1.NC2CCCCC2.C[C@@H]3O[C@H](OP(O)(O)=O)[C@@H](O)[C@H](O)[C@@H]3O
InChI
1S/2C6H13N.C6H13O8P/c2*7-6-4-2-1-3-5-6;1-2-3(7)4(8)5(9)6(13-2)14-15(10,11)12/h2*6H,1-5,7H2;2-9H,1H3,(H2,10,11,12)/t;;2-,3+,4+,5-,6+/m..0/s1
InChI 密鑰
FQMPFZHILABVMA-PEJHDPODSA-N
相關類別
應用
β-L-Fucose 1-phosphate is suitable as both substrate and product to identify, differentiate and characterize GTP fucose pyrophosphorylase(s) (GFPP; fucose-1-phosphate guanylyltransferases) involved in the formation of the nucleotide-sugar GDP-beta-l-fucose and other fucosylation donor substrates such as 3,3′-Diaminobenzidine (GDP)-β-l-fucose. β-L-Fucose 1-phosphate may be used to generate new fucosylation donor substrates for use in glycan fucosylation research.
其他說明
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
Toshihisa Kotake et al.
The Journal of biological chemistry, 283(13), 8125-8135 (2008-01-18)
Monomeric sugars generated during the metabolism of polysaccharides, glycoproteins, and glycolipids are imported to the cytoplasm and converted to respective nucleotide sugars via monosaccharide 1-phosphates, to be reutilized as activated sugars. Because L-fucose (L-Fuc) is activated mainly in the form
Leonie Engels et al.
Glycobiology, 24(2), 170-178 (2013-11-20)
Fucosyltransferases (FucTs) are essential tools for the synthesis of fucosylated glycoconjugates. Multistep enzyme catalysis of fucosylated glycans is not limited as long as isolated and well-characterized FucTs are available. The present paper introduces a novel bacterial α1,2-FucT of the glycosyltransferase
Bing Ma et al.
Glycobiology, 16(12), 158R-184R (2006-09-16)
Fucosylated carbohydrate structures are involved in a variety of biological and pathological processes in eukaryotic organisms including tissue development, angiogenesis, fertilization, cell adhesion, inflammation, and tumor metastasis. In contrast, fucosylation appears less common in prokaryotic organisms and has been suggested
GDP-L-fucose pyrophosphorylase. Purification, cDNA cloning, and properties of the enzyme.
Pastuszak I, Ketchum C, Hermanson G, et al.
The Journal of Biological Chemistry, 4273, 30165-30174 (1998)
Stephen Quirk et al.
Biochemistry, 44(39), 13172-13178 (2005-09-28)
GTP-l-fucose pyrophosphorylase(GFPP) catalyzes the reversible formation of the nucleotide-sugar GDP-beta-l-fucose from guanosine triphosphate and beta-l-fucose-1-phosphate. The enzyme functions primarily in the mammalian liver and kidney to salvage free fucose during the breakdown of glycoproteins and glycolipids. GFPP shares little primary
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務