全部照片(1)
About This Item
經驗公式(希爾表示法):
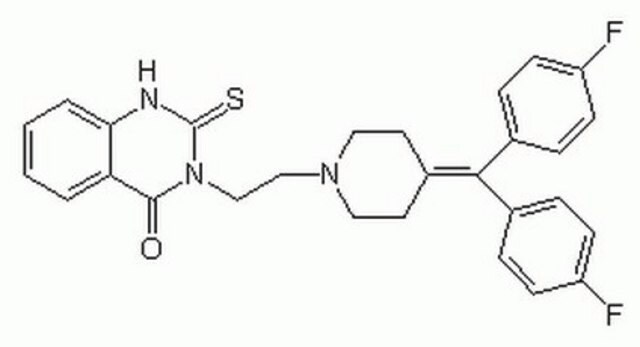
C28H25F2N3OS
CAS號碼:
分子量::
489.58
MDL號碼:
分類程式碼代碼:
41106300
PubChem物質ID:
NACRES:
NA.77
推薦產品
生物源
synthetic (organic)
品質等級
化驗
≥97% (HPLC)
形狀
solid
顏色
pale yellow
mp
228-230 °C
溶解度
0.1 M HCl: slightly soluble
0.1 M NaOH: slightly soluble
DMSO: soluble
H2O: insoluble
ethanol: soluble
ethyl acetate: soluble
儲存溫度
−20°C
SMILES 字串
Fc1ccc(cc1)\C(=C2\CCN(CCN3C(=S)Nc4ccccc4C3=O)CC2)c5ccc(F)cc5
InChI
1S/C28H25F2N3OS/c29-22-9-5-19(6-10-22)26(20-7-11-23(30)12-8-20)21-13-15-32(16-14-21)17-18-33-27(34)24-3-1-2-4-25(24)31-28(33)35/h1-12H,13-18H2,(H,31,35)
InChI 密鑰
ZCNBZFRECRPCKU-UHFFFAOYSA-N
應用
甘油二酯激酶抑制剂II可用于确定嵌合抗原受体 (CAR) 改造的细胞毒性T细胞的肿瘤诱导抑制。它还用于诱导T细胞急性淋巴细胞白血病中的pAkt和PKR样细胞外信号调节激酶 (pErk) 信号。
生化/生理作用
二酰基甘油激酶抑制剂。抑制红细胞膜中[38P]1-油酰基-2-乙酰甘油基-3-磷酸(OAPA)的形成:IC50 = 3.3 μM。
特點和優勢
这种化合物是激酶磷酸酶生物学研究的特色产品。点击此处发现更多特色激酶磷酸酶生物产品。在sigma.com/discover-bsm可了解更多关于生物活性小分子的其他研究领域。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
X Du et al.
The Biochemical journal, 357(Pt 1), 275-282 (2001-06-21)
We have previously shown that unsaturated fatty acids amplify platelet-derived-growth-factor (PDGF)-induced protein kinase C (PKC) activation in vascular smooth-muscle cells (VSMCs). Diacylglycerol-induced PKC activation is normally terminated by diacylglycerol kinases (DGKs). We thus hypothesized that fatty acids act by inhibiting
M Galdiero et al.
The Journal of infection, 46(2), 111-119 (2003-03-14)
In the present study a monocytic cell line, U937, was used to investigate the possible involvement of protein tyrosine kinases (NT-PTKs), protein kinase A (PKA) and protein kinase C (PKC) in cell signaling pathways following Salmonella enterica serovar Typhimurium porin
Y Jiang et al.
Biochemical pharmacology, 59(7), 763-772 (2000-03-16)
Diacylglycerol kinases (DGKs) attenuate diacylglycerol-induced protein kinase C activation during stimulated phosphatidylinositol turnover. This reaction also initiates phosphatidylinositol resynthesis. Two agents, 3-(2-(4-[bis-(4-fluorophenyl)methylene]-1-piperidinyl)ethyl)-2,3-dihydro -2-thioxo-4(1H)quinazolinone (R59949) and 6-(2-(4-[(4-fluorophenyl)phenylmethylene]-1-piperidinyl)ethyl)-7-m ethyl-5H-thiazolo(3,2-a)pyrimidin-5-one (R59022), inhibit diacylglycerol phosphorylation in several systems. To examine the mechanism of this
Multifactorial T-cell hypofunction that is reversible can limit the efficacy of chimeric antigen receptor-transduced human T cells in solid tumors
Moon EK, et al.
Clinical Cancer Research, 20(16), 4262-4273 (2014)
Michelle A Blaskovich et al.
PloS one, 8(10), e78632-e78632 (2013-11-10)
Lysophosphatidic acid acyltransferase (LPAAT-β) is a phosphatidic acid (PA) generating enzyme that plays an essential role in triglyceride synthesis. However, LPAAT-β is now being studied as an important regulator of cell growth and differentiation and as a potential therapeutic target
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務