About This Item
推薦產品
生物源
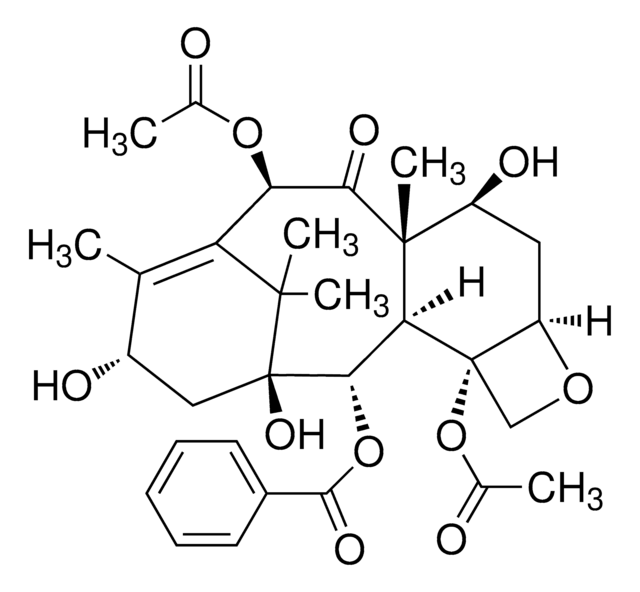
Taxus baccata
品質等級
化驗
≥95% (HPLC)
形狀
solid
分子量
544.59 g/mol
顏色
white
溶解度
methanol: soluble, clear, colorless (5 mg + 0.1 mL MeOH)
抗生素活性譜
neoplastics
作用方式
DNA synthesis | interferes
儲存溫度
2-8°C
SMILES 字串
[H][C@@]12C[C@H](O)[C@@]3(C)C(=O)[C@H](O)C4=C(C)[C@@H](O)C[C@@](O)([C@@H](OC(=O)c5ccccc5)[C@]3([H])[C@@]1(CO2)OC(C)=O)C4(C)C
InChI
1S/C29H36O10/c1-14-17(31)12-29(36)24(38-25(35)16-9-7-6-8-10-16)22-27(5,23(34)21(33)20(14)26(29,3)4)18(32)11-19-28(22,13-37-19)39-15(2)30/h6-10,17-19,21-22,24,31-33,36H,11-13H2,1-5H3/t17-,18-,19+,21+,22-,24-,27+,28-,29+/m0/s1
InChI 密鑰
YWLXLRUDGLRYDR-ZHPRIASZSA-N
尋找類似的產品? 前往 產品比較指南
應用
生化/生理作用
訊號詞
Danger
危險分類
Carc. 1B - Eye Irrit. 2 - Muta. 1B - Skin Irrit. 2 - STOT RE 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務