推薦產品
化驗
≥99%
形狀
solid
SMILES 字串
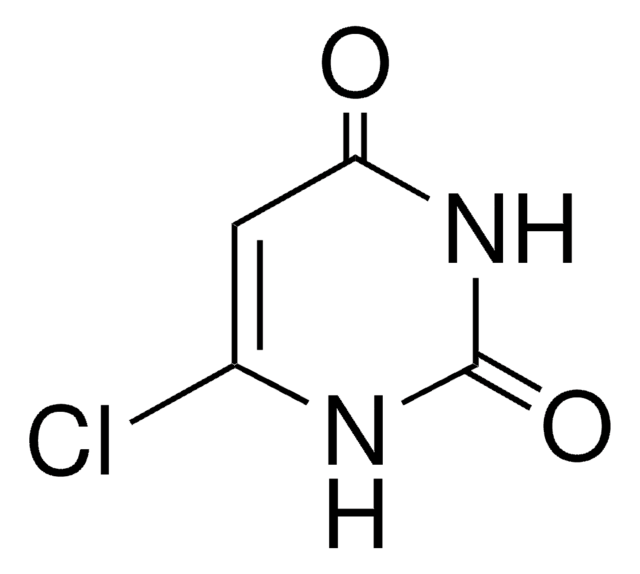
[H]N1C(Cl)=CC(=O)NC1=O
InChI
1S/C4H3ClN2O2/c5-2-1-3(8)7-4(9)6-2/h1H,(H2,6,7,8,9)
InChI 密鑰
PKUFNWPSFCOSLU-UHFFFAOYSA-N
應用
Chlorouracil (4-Chlorouracil; 6-Chlorouracil) is a halogenated uracil that is useful in studies of the effects of halogenation on nucleic acid base-pair stability and alkali metal ion affinity.
Reaction of 6-chlorouracil with 4-(dimethylamino)pyridine, 4-methylpyridine, and pyridin-4-yl-morpholine yielded pyridinium-substituted uracils as chlorides which were converted into pyridinium uracilates by deprotonation. These heterocyclic mesomeric betaines are cross-conjugated and thus possess separate cationic (pyridinium) and anionic (uracilate) moieties. Calculations and X-ray single crystal analyses may be used to characterize these systems and to compare the salts with the betaines.
Reaction of 6-chlorouracil with 4-(dimethylamino)pyridine, 4-methylpyridine, and pyridin-4-yl-morpholine yielded pyridinium-substituted uracils as chlorides which were converted into pyridinium uracilates by deprotonation. These heterocyclic mesomeric betaines are cross-conjugated and thus possess separate cationic (pyridinium) and anionic (uracilate) moieties. Calculations and X-ray single crystal analyses may be used to characterize these systems and to compare the salts with the betaines.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Andreas Schmidt et al.
Organic & biomolecular chemistry, 4(16), 3056-3066 (2006-08-04)
Reaction of 6-chlorouracil with 4-(dimethylamino)pyridine, 4-methylpyridine, and pyridin-4-yl-morpholine yielded pyridinium-substituted uracils as chlorides which were converted into pyridinium uracilates by deprotonation. These heterocyclic mesomeric betaines are cross-conjugated and thus possess separate cationic (pyridinium) and anionic (uracilate) moieties. Calculations and X-ray
Zhibo Yang et al.
Journal of the American Chemical Society, 126(49), 16217-16226 (2004-12-09)
The influence of halogenation on the properties of uracil and its noncovalent interactions with alkali metal ions is investigated both experimentally and theoretically. Bond dissociation energies of alkali metal ion-halouracil complexes, M+(XU), are determined using threshold collision-induced dissociation techniques in
Photochemical transformation of 6-chlorouracil and some alkylated analogues.
Z Kazimierczuk et al.
Biochimica et biophysica acta, 254(2), 157-166 (1971-12-16)
Francesca Bartoccini et al.
Organic & biomolecular chemistry, 10(44), 8860-8867 (2012-10-11)
A small library of 8-substituted 9-deazaxanthines has been prepared by late-stage diversification of an 8-bromo-9-deazaxanthine. By utilizing palladium-catalyzed cross-coupling reactions a single key precursor can be transformed into a variety of 8-substituted-9-deazaxanthine compounds. Three key 8-bromo-9-deazaxanthine intermediates were efficiently prepared
Tumor uptake of radiolabelled pyrimidine bases and pyrimidine nucleosides in animal models--V. 6-[36Cl]chlorouracil, 6-[82Br]bromouracil and 6-[123I]iodouracil.
Y W Lee et al.
International journal of nuclear medicine and biology, 11(3-4), 262-266 (1984-01-01)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務