推薦產品
品質等級
化驗
≥80% (HPLC)
形狀
solid
溶解度
H2O: 50 mg/mL
抗生素活性譜
Gram-negative bacteria
Gram-positive bacteria
作用方式
protein synthesis | interferes
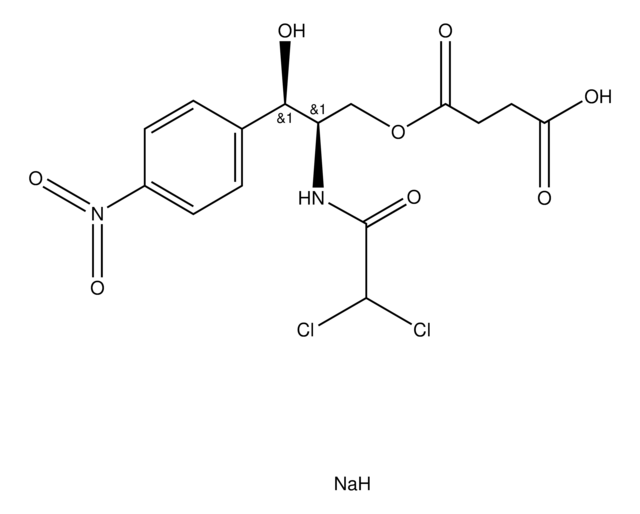
SMILES 字串
[Na].O[C@@H]([C@@H](COC(=O)CCC(O)=O)NC(=O)C(Cl)Cl)c1ccc(cc1)N(=O)=O
InChI
1S/C15H16Cl2N2O8.Na.H/c16-14(17)15(24)18-10(7-27-12(22)6-5-11(20)21)13(23)8-1-3-9(4-2-8)19(25)26;;/h1-4,10,13-14,23H,5-7H2,(H,18,24)(H,20,21);;/t10-,13-;;/m1../s1
InChI 密鑰
RJOAHMNSYANTPN-OWVUFADGSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
Chemical structure: phenicole
應用
Chloramphenicol is often used for bacterial selection in molecular biology applications at 10-20 μg/mL and as a selection agent for transformed cells containing chloramphenicol reistance genes.
生化/生理作用
Chloramphenicol is a synthetic antibiotic, isolated from strains of Streptomyces venezuelae. It inhibits bacterial protein synthesis by blocking the peptidyl transferase step by binding to the 50S ribosomal subunit and preventing attachment of aminoacyl tRNA to the ribosome. It also inhibits mitochondrial and chloroplast protein synthesis and ribosomal formation of (p)ppGpp, de-pressing rRNA transcription.
Mode of Resistance: Use of chloramphenicol acetyltransferase will acetylate the product and inactivate it.
Antimicrobial Spectrum: This is a broad spectrum antibiotic against gram-positive and gram-negative bacteria, and is used mainly for ophthalmic and veterinary purposes.
Mode of Resistance: Use of chloramphenicol acetyltransferase will acetylate the product and inactivate it.
Antimicrobial Spectrum: This is a broad spectrum antibiotic against gram-positive and gram-negative bacteria, and is used mainly for ophthalmic and veterinary purposes.
注意
Stock solutions should be stored at 2-8°C and are stable at 37°C for 5 days. Aqueous solutions are neutral and stable over a wide pH range, with 50% hydrolysis occurring after 290 days. Use of a borax buffered solution reduces this number to 14%. Solutions should be protected from light as photochemical decomposition results in a yellowing of the solution. Heating aqueous solutions at 115°C for 30 minutes results in a 10% loss of chloramphenicol.
準備報告
Stock solutions can be prepared directly in the vial at any recommended concentration. A solution at 50 mg/mL in ethanol yields a clear, very faint, yellow solution. Degradation of chloramphenicol in aqueous solution is catalyzed by general acids and bases. This rate of degradation is independent of the ionic strength and pH.
其他說明
Keep container tightly closed in a dry and well-ventilated place.
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral - Carc. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
個人防護裝備
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
客戶也查看了
G C Allen et al.
Applied and environmental microbiology, 56(4), 1025-1032 (1990-04-01)
Succinate- or oxygen-limited continuous cultures were used to study the influences of different concentrations of dissolved oxygen and ammonia on the growth, respiration, and polypeptide patterns of Bradyrhizobium sp. (Arachis) strain 3G4b20. During succinate-limited growth, molar growth yields on succinate
M W Stinson et al.
Journal of bacteriology, 119(1), 152-161 (1974-07-01)
The ability of succinate to repress the secretion of Pseudomonas lemoignei poly-beta-hydroxybutyrate depolymerase was a function of pH. Repression only occurred when the pH of the medium was 7.0 or less. At a higher pH, lack of sensitivity to succinate
Mode of action of chloramphenicol. III. Action of chloramphenicol on bacterial energy metabolism.
F E HAHN et al.
Journal of bacteriology, 69(2), 215-223 (1955-02-01)
Studies on the mechanism of action of chloramphenicol. I. The conformation of chlioramphenicol in solution.
O JARDETZKY
The Journal of biological chemistry, 238, 2498-2508 (1963-07-01)
Roberta A Gottlieb et al.
Autophagy, 7(4), 434-435 (2010-12-29)
Interventions that reduce infarct size in animal models have largely failed to improve outcome in patients suffering acute myocardial infarction (MI), or 'heart attack'. Our group recently reported a reduction of infarct size by chloramphenicol treatment in a porcine in
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務