全部照片(1)
About This Item
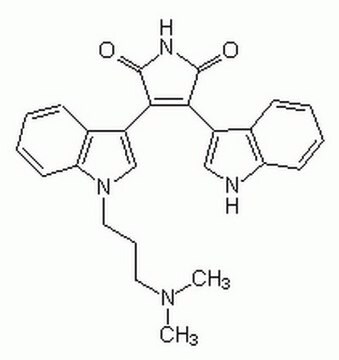
經驗公式(希爾表示法):
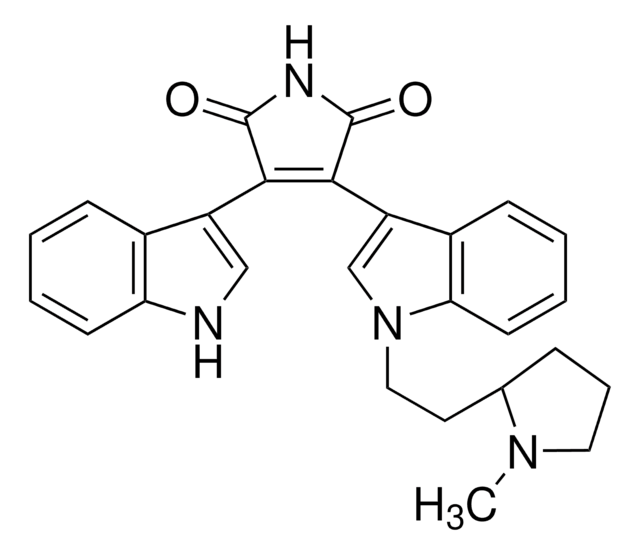
C26H24N4O2 · HCl
CAS號碼:
分子量::
460.96
MDL號碼:
分類程式碼代碼:
12352111
PubChem物質ID:
NACRES:
NA.77
推薦產品
生物源
synthetic (organic)
品質等級
化驗
≥90%
形狀
solid
溶解度
DMSO: soluble
儲存溫度
−20°C
SMILES 字串
Cl.Cn1cc(C2=C(C(=O)NC2=O)c3c4CC(CN)CCn4c5ccccc35)c6ccccc16
InChI
1S/C26H24N4O2.ClH/c1-29-14-18(16-6-2-4-8-19(16)29)23-24(26(32)28-25(23)31)22-17-7-3-5-9-20(17)30-11-10-15(13-27)12-21(22)30;/h2-9,14-15H,10-13,27H2,1H3,(H,28,31,32);1H
InChI 密鑰
IMBOYWXMTUUYGZ-UHFFFAOYSA-N
應用
Bisindolylmaleimide X hydrochloride has been used as a protein kinase C (PKC) inhibitor:
- in chemotaxis assays
- to inhibit protein kinase C and to study its effects on the expression of EGR1, NAB2, ZEBRA, and Rta
- in the culture to study its effects on the expression of the kinase-insert domain-containing receptor (KDR)-B1, protein kinase Cθ (PKCθ)-M1a or Abl-HTa and on cell yield in baculovirus (BV)-infected insect cells
生化/生理作用
Bisindolylmaleimide X hydrochloride/Ro 31-8425, a strong and selective protein kinase C (PKC) inhibitor, can reduce the superoxide burst caused by various agonists in neutrophils. It can suppress the responses induced by cell surface receptors and phorbol esters in T cells. In humans, Ro 31-8425 can prevent neutrophil PKC in vitro with an IC50 of 5nM.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
Jianjiang Ye et al.
Journal of virology, 84(23), 12405-12418 (2010-09-24)
The Epstein-Barr virus (EBV) lytic activator genes bzlf1 and brlf1 are conventionally referred to as immediate-early (IE) genes. However, previous studies showed that the earliest expression of these genes was blocked by cycloheximide when the EBV lytic cycle was induced
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務