推薦產品
產品名稱
Cibacron Blue 3G-A, Dye content ≥55 %
形狀
powder
品質等級
成份
Dye content, ≥55%
顏色
dark blue
溶解度
H2O: 10 mg/mL, blue
應用
diagnostic assay manufacturing
hematology
histology
儲存溫度
room temp
SMILES 字串
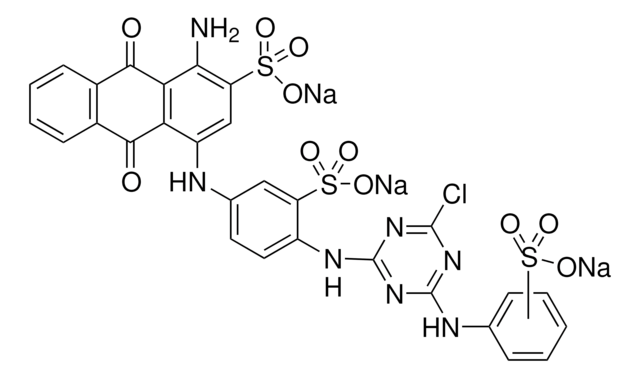
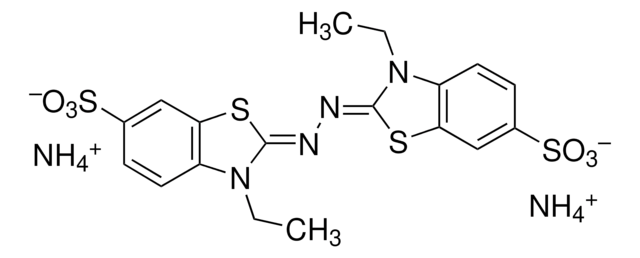
Nc1c(cc(Nc2ccc(Nc3nc(Cl)nc(Nc4ccccc4S(O)(=O)=O)n3)c(c2)S(O)(=O)=O)c5C(=O)c6ccccc6C(=O)c15)S(O)(=O)=O
InChI
1S/C29H20ClN7O11S3/c30-27-35-28(33-16-7-3-4-8-19(16)49(40,41)42)37-29(36-27)34-17-10-9-13(11-20(17)50(43,44)45)32-18-12-21(51(46,47)48)24(31)23-22(18)25(38)14-5-1-2-6-15(14)26(23)39/h1-12,32H,31H2,(H,40,41,42)(H,43,44,45)(H,46,47,48)(H2,33,34,35,36,37)
InChI 密鑰
YKCWQPZFAFZLBI-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
應用
当使用琼脂糖等不溶性多孔支持基质固定时,Cibacron Blue 3GA被用于酶的亲和层析纯化。这种亲和力归因于染料与具有辅因子结合域的蛋白质的天然配体之间的结构相似性。
生化/生理作用
Cibacron Blue 3GA是一种阴离子蒽醌染料。 它是一种P2-嘌呤受体拮抗剂 并且能抑制刺激诱发的大鼠脑皮质组织的谷氨酸释放。Cibacron Blue 3GA能抑制OXA-1 和OXA-2β-内酰胺酶 ,并被用于通过监测色氨酸荧光观察配体与OXA-1 β-内酰胺酶的结合。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
從最近期的版本中選擇一個:
分析證明 (COA)
Lot/Batch Number
C Monaghan et al.
The Biochemical journal, 205(2), 413-417 (1982-08-01)
Although beta-lactamases do not require any nucleotide co-substrates, the OXA-2 type is inhibited competitively by Cibacron Blue 3GA, and by other anthraquinone dyes, including some simpler compounds with no side chain. The enzyme causes a red shift in the spectrum
G C Bennett et al.
British journal of pharmacology, 131(3), 617-623 (2000-10-04)
Evidence has previously been presented that P1 receptors for adenosine, and P2 receptors for nucleotides such as ATP, regulate stimulus-evoked release of biogenic amines from nerve terminals in the brain. Here we investigated whether adenosine and nucleotides exert presynaptic control
Kyle D Schneider et al.
Biochemistry, 48(26), 6136-6145 (2009-06-03)
Class D beta-lactamases hydrolyze beta-lactam antibiotics by using an active site serine nucleophile to form a covalent acyl-enzyme intermediate and subsequently employ water to deacylate the beta-lactam and release product. Class D beta-lactamases are carboxylated on the epsilon-amino group of
S T Thompson et al.
Proceedings of the National Academy of Sciences of the United States of America, 72(2), 669-672 (1975-02-01)
A procedure is described to utilize blue dextran-Sepharose as an affinity chromatographic column specific for the super-secondary structure called the dinucleotide fold, which forms the binding sites for substrates and effectors on a wide range of proteins. The procedure can
I von Kügelgen et al.
British journal of pharmacology, 113(3), 815-822 (1994-11-01)
1. Some postganglionic sympathetic axons possess P2Y-like P2-purinoceptors which, when activated, decrease the release of noradrenaline. We examined the question of whether such receptors also occur at the noradrenergic axons in the rat brain cortex. Slices of the brain cortex
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務