推薦產品
生物源
synthetic or plant
品質等級
化驗
≥98% (HPLC)
形狀
powder
光學活性
[α]25/D -66.0 to -62.0 °, c = 3% (w/v) in water
技術
HPLC: suitable
顏色
light yellow
mp
195 °C
溶解度
water: 50 mg/mL, clear to very slightly hazy, colorless to faintly yellow
儲存溫度
room temp
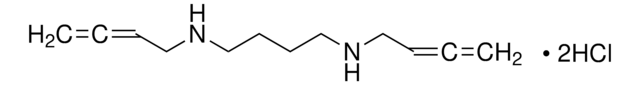
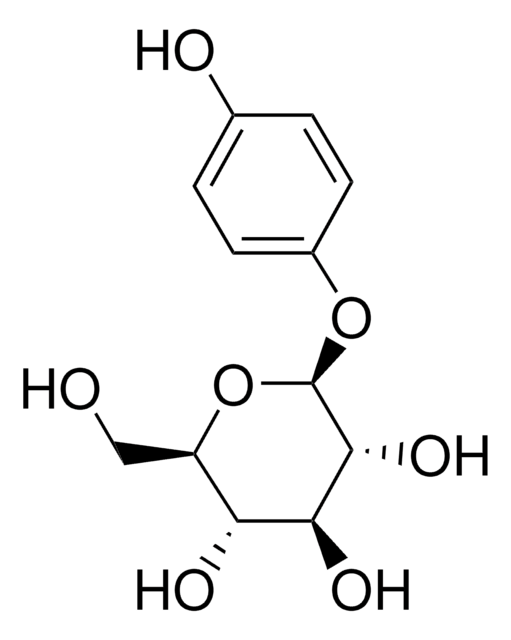
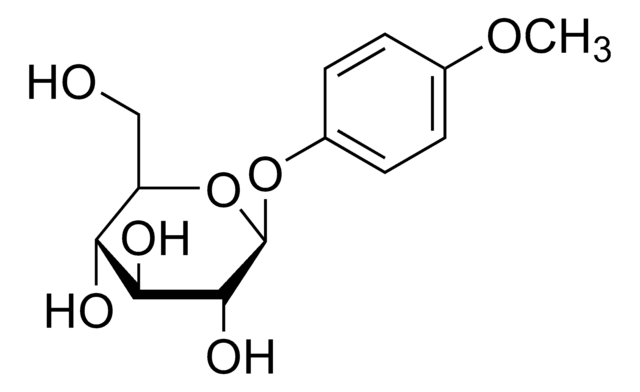
SMILES 字串
OC[C@H]1O[C@@H](Oc2ccc(O)cc2)[C@H](O)[C@@H](O)[C@@H]1O
InChI
1S/C12H16O7/c13-5-8-9(15)10(16)11(17)12(19-8)18-7-3-1-6(14)2-4-7/h1-4,8-17H,5H2/t8-,9-,10+,11-,12-/m1/s1
InChI 密鑰
BJRNKVDFDLYUGJ-RMPHRYRLSA-N
尋找類似的產品? 前往 產品比較指南
相關類別
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
客戶也查看了
Sang Mi An et al.
Phytotherapy research : PTR, 24(8), 1175-1180 (2010-01-16)
Tyrosinase (TYR) catalyzes rate-limiting steps of melanogenesis and thus its inhibitors are potentially useful as hypopigmenting agents. Recently, p-coumaric acid (p-CA) has been suggested to interfere with the pro-melanogenic actions of tyrosine due to its structural similarity with tyrosine (An
Hyo-Jong Lee et al.
Inflammation research : official journal of the European Histamine Research Society ... [et al.], 61(8), 817-825 (2012-04-11)
Arbutin, which is found in the genus Arctostaphylos, is an anti-oxidant and a depigmenting agent. The present study was designed to validate the anti-inflammatory effect of arbutin. The anti-inflammatory properties of arbutin were studied using a lipopolysaccharide (LPS)-stimulated murine BV2
Mi Jeong Kang et al.
Archives of pharmacal research, 34(4), 687-693 (2011-05-06)
A possible role of metabolism by intestinal bacteria in arbutin-induced toxicity was investigated in mammalian cell cultures. Following an incubation of arbutin with intestinal bacteria, either Bifidobacterium longum HY81 or Bifidobacterium adolescentis, for 24 h, its aglycone hydroquinone could be
Shinji Sakuma et al.
Molecular pharmaceutics, 9(4), 922-929 (2012-02-23)
A carboxyl group-terminated polyamidoamine dendrimer (generation: 3.0) bearing arbutin, which is a substrate of Na⁺/glucose cotransporter 1 (SGLT1), via a nonbiodegradable ω-amino triethylene glycol linker (PAMAM-ARB), inhibits SGLT1-mediated D-glucose uptake, as does phloridzin, which is a typical SGLT1 inhibitor. Here
Chunqiao Liu et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 925, 104-109 (2013-04-02)
α-Arbutin is a glycosylated hydroquinone which has inhibitory function against tyrosinase. In this work, a one-step isolation of α-arbutin from Xanthomonas CGMCC 1243 fermentation broth by macroporous resin adsorption chromatography was investigated. The research results indicated that S-8 resin offered
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務





![[D-Phe7]-MSH α](/deepweb/assets/sigmaaldrich/product/images/293/162/6080d768-4eea-4028-b4cf-909d89087b50/640/6080d768-4eea-4028-b4cf-909d89087b50.jpg)