推薦產品
品質等級
化驗
≥98.0% (HPLC)
光學活性
[α]/D -65±3°, c = 1 in chloroform
mp
~258 °C (dec.)
應用
metabolomics
vitamins, nutraceuticals, and natural products
儲存溫度
2-8°C
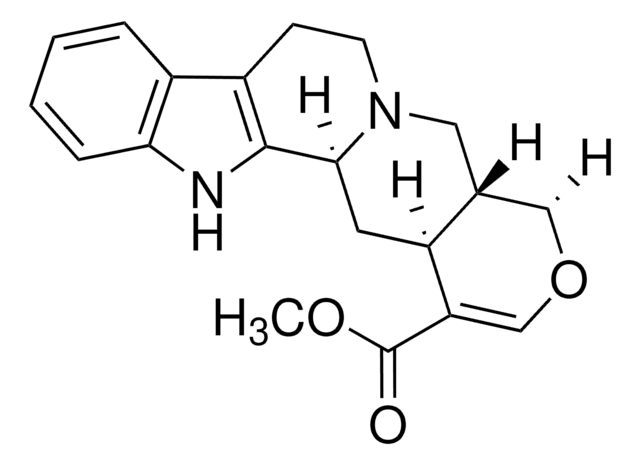
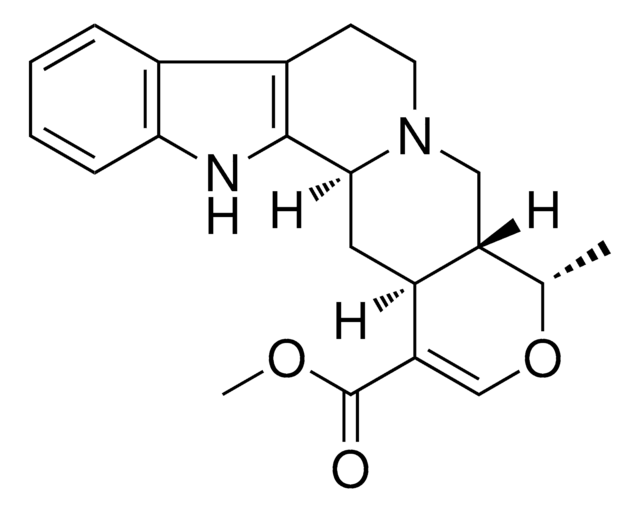
SMILES 字串
N21[C@@H](C[C@H]5[C@@H]([C@@H](OC=C5C(=O)OC)C)C2)c3[nH]c4c(c3CC1)cccc4
InChI
1S/C21H24N2O3/c1-12-16-10-23-8-7-14-13-5-3-4-6-18(13)22-20(14)19(23)9-15(16)17(11-26-12)21(24)25-2/h3-6,11-12,15-16,19,22H,7-10H2,1-2H3/t12-,15-,16+,19-/m0/s1
InChI 密鑰
GRTOGORTSDXSFK-XJTZBENFSA-N
尋找類似的產品? 前往 產品比較指南
應用
Ajmalicine (δ-Yohimbine, Py-Tetrahydroserpentine, Raubasine) is an alkaloid used to study its effects as an antagonist of adrenergic and nicotinic receptors.
生化/生理作用
Metabolite in the indole alkaloid biosynthesis (serpentine production); found naturally in various plants such as Rauwolfia spp., Catharanthus roseus, and Mitragyna speciosa. It shows antimicrobial activity, and is used as an anti-hypertensive and sedative.
包裝
Bottomless glass bottle. Contents are inside inserted fused cone.
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 2 Oral
儲存類別代碼
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
W G Kurz et al.
Planta medica, 42(1), 22-31 (1981-05-01)
A cell line of Catharanthus roseus (L.) G. Don coded PRL # 200, was characterized with respect to its biosynthetic capabilities for indolealkaloids, in particular catharanthine, in suspension cultures. Other alkaloids isolated are vallesiachotamine isomers, ajmalicine, hörhammericine, hörhammerinine, vindolinine, 19-epivindolinine
Francisco León et al.
Natural product communications, 4(7), 907-910 (2009-09-08)
Mitragyna speciosa (Rubiaceae) has traditionally been used in the tropical regions of Asia, Africa and Indonesia as a substitute for opium. Indole alkaloids are the most common compounds that have been isolated. We investigated the constituents of the leaves of
P Demichel et al.
British journal of pharmacology, 83(2), 505-510 (1984-10-01)
The actions of raubasine, tetrahydroalstonine and akuammigine were studied on pre- and postsynaptic alpha-adrenoceptors of the rat vas deferens. These three drugs competitively antagonized the effect of noradrenaline on postsynaptic alpha-adrenoceptors, yielding pA2 values of 6.57, 4.56 and 4.68 respectively.
David M Pereira et al.
Phytomedicine : international journal of phytotherapy and phytopharmacology, 17(8-9), 646-652 (2009-12-08)
The leaves of Catharanthus roseus constitute the only source of the well known indolomonoterpenic alkaloids vincristine and vinblastine. In this work we studied the biological potential of the roots, which are used in several countries as decocts or hot water
Roberts, M. F.
Alkaloids: biochemistry, ecology, and medicinal applications, 450-450 (1998)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務