推薦產品
化驗
≥95.0% (HPLC)
形狀
powder
應用
metabolomics
vitamins, nutraceuticals, and natural products
SMILES 字串
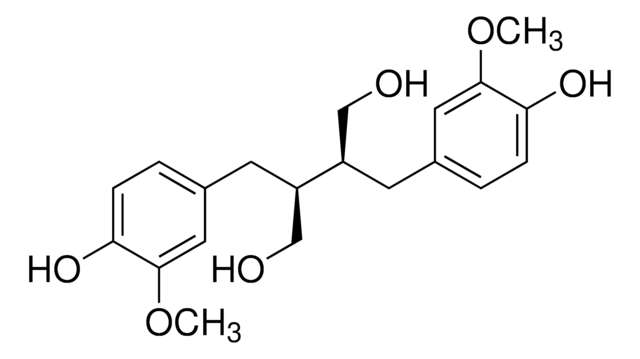
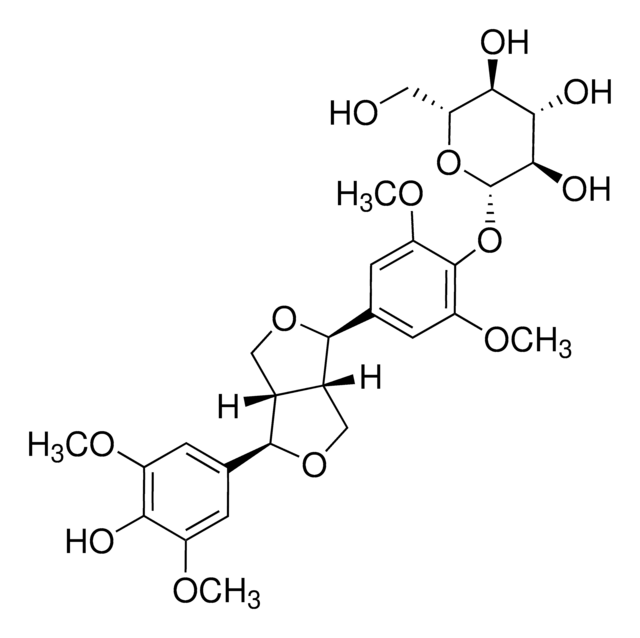
COc1cc(C[C@H]2CO[C@@H]([C@H]2CO)c3ccc(O)c(OC)c3)ccc1O
InChI
1S/C20H24O6/c1-24-18-8-12(3-5-16(18)22)7-14-11-26-20(15(14)10-21)13-4-6-17(23)19(9-13)25-2/h3-6,8-9,14-15,20-23H,7,10-11H2,1-2H3/t14-,15-,20+/m0/s1
InChI 密鑰
MHXCIKYXNYCMHY-AUSJPIAWSA-N
一般說明
落叶松树脂醇(LA)是一种膳食植物雌激素植物木脂素。它是许多传统草药植物和谷物中主要的活性植物化学成分之一。
應用
落叶松树脂醇可作为参考标准品:
- 用于木脂素植物雌激素和木脂素的纯化、鉴定和分析
- 使用高效液相色谱法(HPLC)测定松脂醇还原为落叶松树脂醇的对映体成分。
- 使用超高效液相色谱法(UPLC)对七种黑小麦球状物中的木脂素进行定性和定量分析。
生化/生理作用
落叶松树脂醇是一种强效膳食抗氧化剂,可通过潜在螯合催化Fe2+离子来清除自由基。它对食源性病原体金黄色葡萄球菌和大肠杆菌O157:H7具有抗菌效果。落叶松树脂醇是一种有效抗癌剂,可在体外抑制人HepG2细胞增殖和诱导凋亡。此外,它体内和体外研究中分别表现出抗炎和抗糖尿病的效果。
包裝
无底玻璃瓶。内含物装在锥底内插管中。
訊號詞
Warning
危險聲明
危險分類
Aquatic Acute 1
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
客戶也查看了
Niina M Saarinen et al.
International journal of cancer, 123(5), 1196-1204 (2008-06-06)
Lariciresinol is a dietary lignan that accounts for a significant portion of the total phytoestrogen intake from Western foods. Recent epidemiological studies suggest that high dietary intake of lignans and lariciresinol is associated with reduced breast cancer risk. However, no
Bomi Hwang et al.
Biochemical and biophysical research communications, 410(3), 489-493 (2011-06-18)
Lariciresinol is an enterolignan precursor isolated from the herb Sambucus williamsii, a folk medicinal plant used for its therapeutic properties. In this study, the antifungal properties and mode of action of lariciresinol were investigated. Lariciresinol displays potent antifungal properties against
Hisashi Nishiwaki et al.
Journal of agricultural and food chemistry, 59(24), 13089-13095 (2011-11-10)
All stereoisomers of lariciresinol were synthesized to examine the effect of stereochemistry on plant growth. Configuration of benzylic 7-positions was constructed through S(N)1 or S(N)2 intramolecular etherification. 8- and 8'-position configurations were established from the starting material except for all
Simone Angeloni et al.
Journal of mass spectrometry : JMS, 53(9), 842-848 (2018-06-21)
Lignans are polyphenolic compounds that are considered phytoestrogens for their plant origins, and they possess different biological activities. Three different extraction methods, ie, "dilute and shoot", acidic hydrolysis, and enzymatic digestion, have been compared for extracting lignans (secoisolariciresinol (SECO), matairesinol
Heidi Schwartz et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 838(2), 78-85 (2006-06-06)
The paper describes a method for the determination of selected lignans in plant foods. First, samples were submitted to methanolysis resulting in cleavage of ester bonds between lignan glycosides and organic acids. Glycosidic linkages were then broken by enzymatic hydrolysis
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務