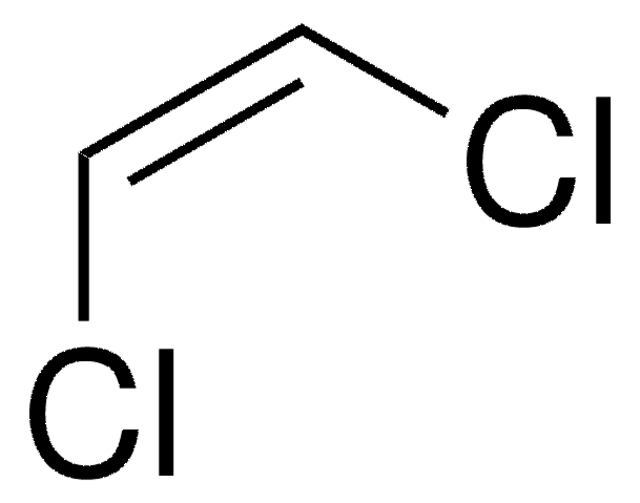
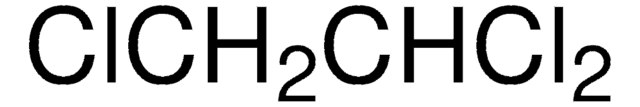推薦產品
等級
ACS reagent
品質等級
蒸汽密度
5.83 (vs air)
蒸汽壓力
13 mmHg ( 20 °C)
19 mmHg ( 25 °C)
化驗
≥99.0%
形狀
liquid
技術
FTIR: suitable
雜質
≤0.05% water
蒸發殘留物
≤0.0005%
顏色
APHA: ≤10
折射率
n20/D 1.505 (lit.)
bp
121 °C (lit.)
mp
−22 °C (lit.)
溶解度
water: soluble 0.15 g/L at 25 °C
密度
1.623 g/mL at 25 °C (lit.)
SMILES 字串
Cl\C(Cl)=C(\Cl)Cl
InChI
1S/C2Cl4/c3-1(4)2(5)6
InChI 密鑰
CYTYCFOTNPOANT-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
Tetrachloroethylene (perchloroethylene, PCE), is a chlorinated ethylene compound commonly used as a dry cleaning and degreasing solvent. It shows IR transparency as it has no C–H bonds making it an ideal solvent for IR spectroscopy. PCE is a man-made pollutant which is difficult to degrade. It is a ground water contaminant which has adverse effect on human health due to its potential toxicity and carcinogenicity. Some of the methods proposed for its degradation are Fenton oxidation treatment, reductive dehalogenation under methanogenic condition, and reduction using zero valent metal ions. One of the methods reported for its synthesis is by reacting ethylene dichloride and chlorine.
應用
Tetrachloroethylene may be used as a film-forming electrolyte additive in the manufacture of lithium ion batteries. It may also be used as an extractant for the estimation of oil and grease in water by Fourier transform infrared spectroscopy (FT-IR).
訊號詞
Warning
危險分類
Aquatic Chronic 2 - Carc. 2 - Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
標靶器官
Central nervous system
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
No data available
閃點(°C)
No data available
客戶也查看了
Validation of a FT-IR method for the determination of oils and grease in water using tetrachloroethylene as the extraction solvent.
Farmaki E, et al.
Desalination, 210(1), 52-60 (2007)
Tetrachloroethylene as new film-forming additive to propylene carbonate-based electrolytes for lithium ion batteries with graphitic anode.
Hu Y, et al.
Solid State Ionics, 176(1), 53-56 (2005)
Decomposition of aqueous tetrachloroethylene by Fenton oxidation treatment.
Yoshida M, et al.
Water Science and Technology, 42(1-2), 203-208 (2000)
Robert DM and Murphy B.
Chlorinated Solvents: A Forensic Evaluation, 84-85 (2013)
D Ryoo et al.
Nature biotechnology, 18(7), 775-778 (2000-07-11)
Tetrachloroethylene (PCE) is thought to have no natural source, so it is one of the most difficult contaminants to degrade biologically. This common groundwater pollutant was thought completely nonbiodegradable in the presence of oxygen. Here we report that the wastewater
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務