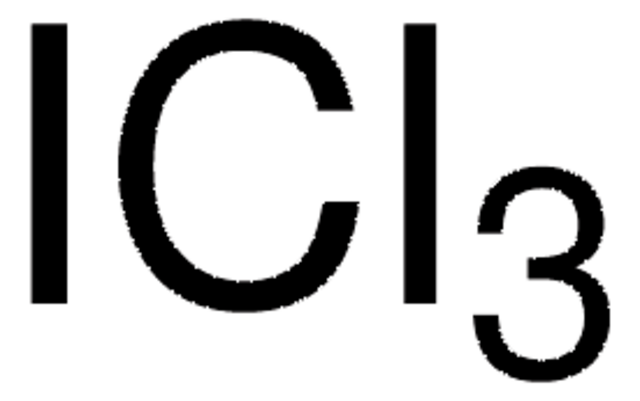推薦產品
等級
ACS reagent
品質等級
形狀
solid or liquid
雜質
≤0.005% insolubles
bp
97.4 °C (lit.)
溶解度
acetic acid: soluble(lit.)
acetone: soluble(lit.)
alcohol: soluble(lit.)
carbon disulfide: soluble(lit.)
密度
3.24 g/mL at 25 °C (lit.)
儲存溫度
2-8°C
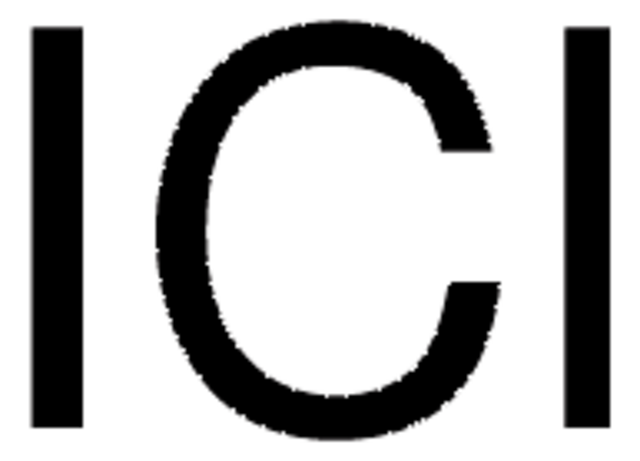
SMILES 字串
ClI
InChI
1S/ClI/c1-2
InChI 密鑰
QZRGKCOWNLSUDK-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
Iodine monochloride is an interhalogen compound. It forms various complexes with ethyl, isopropyl and t-butylbenzenes. Equilibrium constants for the formation of these complexes have been evaluated. It affords electrically conducting solution on dissolution in polar solvents. Its reaction with thymidine, 3-mono- and 3,3′,5′-trialkylsubstitued thymidine showed that it helps in deglycosylation, anomerization and isomerization of thymidine.
應用
Iodine monochloride (ICl) may be employed for the halogenation of methoxy and dimethoxybenzenes. It may be used for the synthesis of flavones.
Iodine monochloride may be used in the synthesis of the following:
- 2-(4-haloisoquinolin-1-yl)ethanol derivatives
- 5-iodosalicylaldehydes
- 5-aryl-6-iodo-8-phenylpyrazolo[1,5-c]-1,2,4-triazolo[4,3-a]pyrimidines
- (±)-1-cyclohexyl-4-iodo-3-methoxybutan-1-ol
- (±)-4-Iodo-3-methoxy-1-phenylbutan-1-ol
訊號詞
Danger
危險聲明
危險分類
Eye Dam. 1 - Skin Corr. 1B - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
Brisbois RG, et al.
e-EROS Encyclopedia of Reagents for Organic Synthesis. (2013)
Synthesis and Electrophilic Substitutions of Novel Pyrazolo [1, 5-c]-1, 2, 4-triazolo [4, 3-a] pyrimidines.
Atta KFM.
Molecules (Basel), 16(8), 7081-7096 (2011)
Eric Stefan et al.
Tetrahedron, 69(36), 7706-7712 (2013-09-24)
Ether transfer methodology is capable of stereoselectively generating 1,3-diol mono- and diethers in good yield. Surprisingly, allylic and benzylic substrates provide none of the desired products when exposed to previously optimized conditions of iodine monochloride. Herein, second-generation activation conditions for
Synthesis of Luminescent Ethynyl-Extended Regioisomers of Borate Complexes Based on 2-(2'-Hydroxyphenyl) benzoxazole.
23 Massue J, et al.
Chemistry (Weinheim An Der Bergstrasse, Germany), 19(17), 5375-5386 (2013)
Eagleson M.
Concise Encyclopedia Chemistry, 477-477 (1994)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務