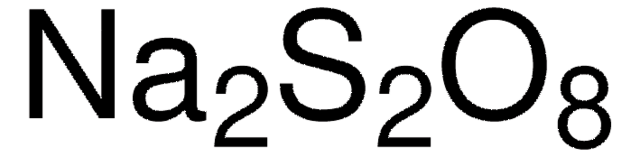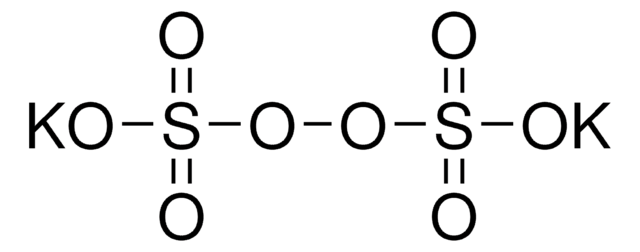推薦產品
等級
reagent grade
品質等級
化驗
≥98%
形狀
powder, crystals or granules
反應適用性
reagent type: oxidant
SMILES 字串
[Na+].[Na+].[O-]S(=O)(=O)OOS([O-])(=O)=O
InChI
1S/2Na.H2O8S2/c;;1-9(2,3)7-8-10(4,5)6/h;;(H,1,2,3)(H,4,5,6)/q2*+1;/p-2
InChI 密鑰
CHQMHPLRPQMAMX-UHFFFAOYSA-L
尋找類似的產品? 前往 產品比較指南
一般說明
过硫酸钠,又称过氧二硫酸钠,是一种无机化合物,是一种水溶性强氧化剂,稳定性好,易于处理。它通常用作有机合成反应的氧化剂,如过渡金属催化反应或无金属反应。此外,它还被用作聚合引发剂和通过热活化分解合成硫酸盐自由基阴离子。
應用
过硫酸钠可用作氧化剂:
- 2-炔基苯并腈与芳基亚磺酸钠的自由基环化级联反应,以合成磺化茚酮。
- 通过二氧化硅负载的氯化铝催化 Baeyer-Villiger 氧化将环和无环酮转化为内酯或酯。
訊號詞
Danger
危險分類
Acute Tox. 4 Oral - Ox. Sol. 3 - Resp. Sens. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
5.1B - Oxidizing hazardous materials
水污染物質分類(WGK)
WGK 1
閃點(°F)
Not applicable
閃點(°C)
Not applicable
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
客戶也查看了
Esther Eljarrat-Binstock et al.
Investigative ophthalmology & visual science, 45(8), 2543-2548 (2004-07-28)
To assess the corneal iontophoretic delivery of gentamicin by drug-loaded hydrogel probe, and to determine the resultant ocular disposition and elimination of the drug from the cornea and anterior chamber. Corneal iontophoresis of gentamicin sulfate was studied in healthy white
N Sabri et al.
The Science of the total environment, 427-428, 382-389 (2012-05-15)
The objective of this work was to evaluate the removal of ibuprofen (IBP) using the oxidants hydrogen peroxide (H(2)O(2)) and sodium persulfate (Na(2)S(2)O(8)). The ability of magnetite (Fe(3)O(4)) to activate persulfate (PS) and H(2)O(2) for the oxidation of IBP at
Mushtaque Ahmad et al.
Journal of contaminant hydrology, 115(1-4), 34-45 (2010-05-05)
Persulfate dynamics in the presence of subsurface minerals was investigated as a basis for understanding persulfate activation for in situ chemical oxidation (ISCO). The mineral-mediated decomposition of persulfate and generation of oxidants and reductants was investigated with four iron and
Aikaterini Tsitonaki et al.
Water research, 42(4-5), 1013-1022 (2007-10-19)
The effects of heat-activated persulfate on indigenous microorganisms and microcosms augmented with Pseudomonas putida KT2440 were studied in laboratory batch reactors with aquifer material. Microscopic enumeration was used to measure the changes in cell density, and acetate consumption was used
Richard L Johnson et al.
Environmental science & technology, 42(24), 9350-9356 (2009-01-30)
Contaminant destruction with in situ chemical oxidation (ISCO) using persulfate (peroxydisulfate, S2O8(2-)) can be enhanced by activation, which increases the rate of persulfate decomposition to sulfate radicals (SO4*-). This step initiates a chain of radical reactions involving species (including SO4*-
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務