推薦產品
等級
pharmaceutical primary standard
API 家族
dutasteride
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
2-8°C
SMILES 字串
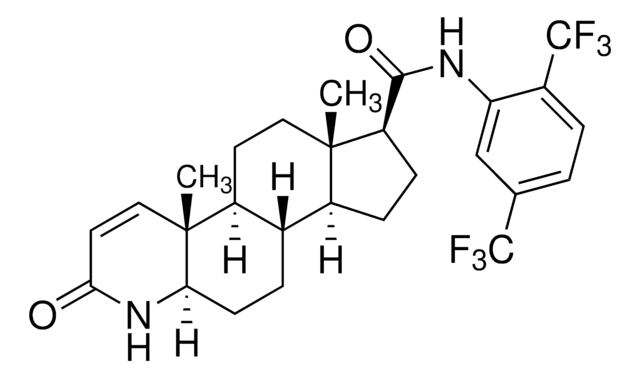
O=C1C=C[C@@]2(C)[C@](CC[C@]3([H])[C@]2([H])CC[C@@]4(C)[C@@]3([H])CC[C@@H]4C(NC5=CC(C(F)(F)F)=CC=C5C(F)(F)F)=O)([H])N1
InChI
1S/C27H30F6N2O2/c1-24-11-9-17-15(4-8-21-25(17,2)12-10-22(36)35-21)16(24)6-7-19(24)23(37)34-20-13-14(26(28,29)30)3-5-18(20)27(31,32)33/h3,5,10,12-13,15-17,19,21H,4,6-9,11H2,1-2H3,(H,34,37)(H,35,36)/t15-,16-,17-,19+,21+,24-,25+/m0/s1
InChI 密鑰
JWJOTENAMICLJG-QWBYCMEYSA-N
基因資訊
human ... SRD5A1(6715) , SRD5A2(6716) , SRD5A3(79644)
尋找類似的產品? 前往 產品比較指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Dutasteride EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
相關產品
產品號碼
描述
訂價
訊號詞
Danger
危險聲明
危險分類
Carc. 2 - Repr. 1B
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
Gerald L Andriole et al.
Urology, 84(2), 393-399 (2014-06-12)
To explore explanations for the numerical imbalance of biopsy-detected Gleason 8-10 prostate cancers (PCa) diagnosed in years 3-4 in the dutasteride and placebo groups of the Reduction by Dutasteride of Prostate Cancer Events (REDUCE) study. REDUCE was a 4-year, randomized
Elahe A Mostaghel et al.
PloS one, 9(10), e111545-e111545 (2014-10-31)
Factors influencing differential responses of prostate tumors to androgen receptor (AR) axis-directed therapeutics are poorly understood, and predictors of treatment efficacy are needed. We hypothesized that the efficacy of inhibiting DHT ligand synthesis would associate with intra-tumoral androgen ratios indicative
Laurence Klotz et al.
Canadian Urological Association journal = Journal de l'Association des urologues du Canada, 8(11-12), E789-E794 (2014-12-09)
We studied the effect of dutasteride on the length of the off-treatment period in prostate cancer patients on intermittent androgen deprivation (IAD) therapy. We conducted a randomized, placebo-controlled Phase II trial in men with localized prostate cancer and a rising
Matthias Oelke et al.
World journal of urology, 32(5), 1133-1140 (2014-05-09)
The purpose of the study was to assess the impact of dutasteride plus tamsulosin combination therapy, compared with dutasteride or tamsulosin monotherapy, on nocturia in men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia (LUTS/BPH) using data from
Adriana C Vidal et al.
Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology, 23(12), 2936-2942 (2014-09-30)
Studies suggest that obesity is associated with lower risk of prostate cancer but more aggressive cancers. As obesity lowers PSA levels, these observations may be influenced by detection bias. We examined the association between obesity and risk of low- and
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務