Y0001510
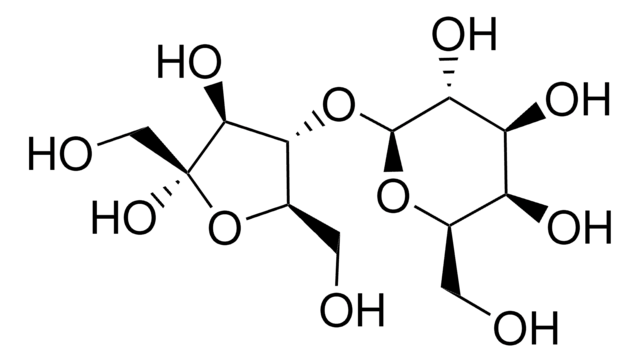
Lactulose for peak identification
European Pharmacopoeia (EP) Reference Standard
同義詞:
Lactulose, 4-O-β-D-Galactopyranosyl-D-fructofuranose, 4-O-β-D-Galactopyranosyl-D-fructose
登入查看組織和合約定價
全部照片(1)
About This Item
經驗公式(希爾表示法):
C12H22O11
CAS號碼:
分子量::
342.30
Beilstein:
93773
MDL號碼:
分類程式碼代碼:
41116107
PubChem物質ID:
NACRES:
NA.24
推薦產品
等級
pharmaceutical primary standard
API 家族
lactulose
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
2-8°C
SMILES 字串
OC[C@H]1O[C@@H](O[C@@H]2[C@@H](CO)O[C@@](O)(CO)[C@H]2O)[C@H](O)[C@@H](O)[C@H]1O
InChI
1S/C12H22O11/c13-1-4-6(16)7(17)8(18)11(21-4)22-9-5(2-14)23-12(20,3-15)10(9)19/h4-11,13-20H,1-3H2/t4-,5-,6+,7+,8-,9-,10+,11+,12+/m1/s1
InChI 密鑰
JCQLYHFGKNRPGE-WJONTELPSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Lactulose for peak identification EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Rifaximin for episodic, overt hepatic encephalopathy: the data are catching up to clinical practice, but questions remain.
Stephen E Congly et al.
The American journal of gastroenterology, 109(4), 598-598 (2014-04-05)
B Vogt et al.
Scandinavian journal of gastroenterology. Supplement, 222, 100-101 (1997-01-01)
The introduction of lactulose as a new therapeutic agent for treatment of hepatic encephalopathy was a major breakthrough in this field. It was hypothesized that lactulose might prevent postoperative renal impairment after biliary surgery in patients with obstructive jaundice. The
F L Weber
Digestive diseases (Basel, Switzerland), 14 Suppl 1, 53-63 (1996-01-01)
Lactulose is the most frequently utilized agent in the treatment of hepatic encephalopathy because of its efficacy and the fact that it has few serious side effects. How this nonabsorbable disaccharide works has been a matter of controversy, but evidence
Lactulose in clinical use.
J Ray et al.
Tropical gastroenterology : official journal of the Digestive Diseases Foundation, 17(2), 12-14 (1996-04-01)
S Salminen et al.
Scandinavian journal of gastroenterology. Supplement, 222, 45-48 (1997-01-01)
During the fermentation of lactulose, short-chain fatty acids are formed with consequent lowering of the colon pH and modification of the microflora. Lactulose promotes the growth of lactic acid bacteria and bifidobacteria and, more specifically, Lactobacillus acidophilus in the colon.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務