推薦產品
等級
pharmaceutical primary standard
API 家族
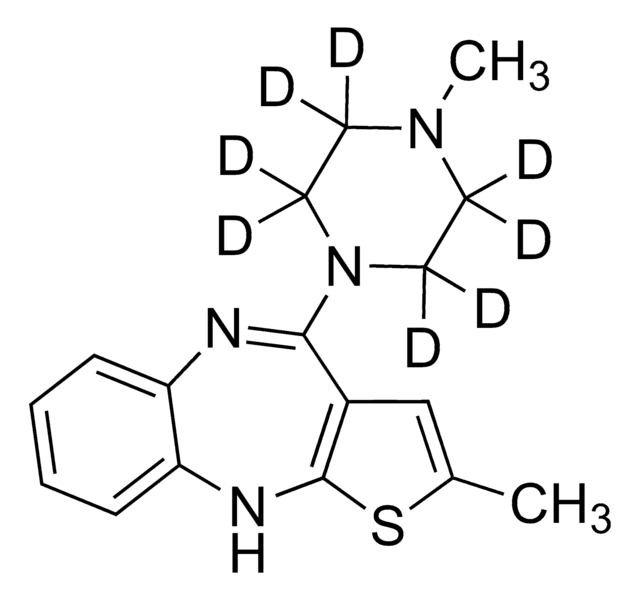
olanzapine
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
2-8°C
SMILES 字串
CN1CCN(CC1)C2=Nc3ccccc3Nc4sc(C)cc24
InChI
1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,19H,7-10H2,1-2H3
InChI 密鑰
KVWDHTXUZHCGIO-UHFFFAOYSA-N
基因資訊
human ... DRD2(1813) , DRD3(1814) , DRD4(1815) , HTR2A(3356) , HTR2C(3358)
尋找類似的產品? 前往 產品比較指南
相關類別
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Olanzapine EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
相關產品
產品號碼
描述
訂價
訊號詞
Danger
危險分類
Acute Tox. 3 Oral - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
標靶器官
Central nervous system
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
客戶也查看了
Jacqueline Flank et al.
Pediatric blood & cancer, 62(3), 496-501 (2014-10-21)
This retrospective review provides preliminary data regarding the safety and efficacy of olanzapine for chemotherapy-induced vomiting (CIV) control in children. Children <18 years old who received olanzapine for acute chemotherapy-induced nausea and vomiting (CINV) control from December 2010 to August
Hirotake Hida et al.
Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology, 40(3), 601-613 (2014-08-15)
Blonanserin differs from currently used serotonin 5-HT₂A/dopamine-D₂ receptor antagonists in that it exhibits higher affinity for dopamine-D₂/₃ receptors than for serotonin 5-HT₂A receptors. We investigated the involvement of dopamine-D₃ receptors in the effects of blonanserin on cognitive impairment in an
Susan L McElroy et al.
Current psychiatry reports, 17(5), 35-35 (2015-03-23)
Psychopharmacologic treatment is playing a greater role in the management of patients with eating disorders. In this paper, we review randomized, placebo-controlled trials (RCTs) conducted in anorexia nervosa (AN), bulimia nervosa (BN), binge eating disorder (BED), and other eating disorders
Erin Schwenger et al.
Clinical pharmacokinetics, 50(7), 415-428 (2011-06-10)
Olanzapine, a second-generation antipsychotic, is a first-line agent in the treatment of schizophrenia. The objective of this review was to determine whether olanzapine warrants clinical pharmacokinetic monitoring in patients with schizophrenia, using a previously published decision-making algorithm. Although olanzapine is
Frantiescoli A Dimer et al.
Journal of biomedical nanotechnology, 10(6), 1137-1145 (2014-04-23)
Olanzapine is an atypical antipsychotic drug, whose chronic use has been associated with the development of potential adverse effects such as weight gain and cardio-metabolic disorders like hypercholesterolemia and diabetes. To circumvent these side effects, the controlled release of olanzapine
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務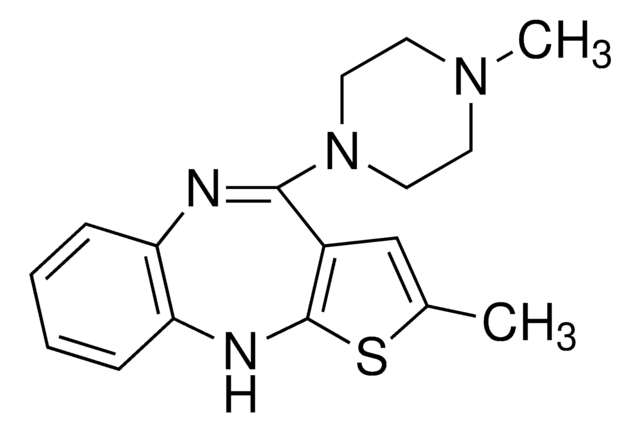
![1-(Chloromethyl)-1-methyl-4-(2-methyl-10H-thieno[2,3-b][1,5]benzodiazepin-4-yl)piperazin-1-ium chloride certified reference material, TraceCERT®, Manufactured by: Sigma-Aldrich Production GmbH, Switzerland](/deepweb/assets/sigmaaldrich/product/images/212/356/1983c6c4-5169-422a-b285-653c093fe48e/640/1983c6c4-5169-422a-b285-653c093fe48e.jpg)