全部照片(1)
About This Item
經驗公式(希爾表示法):
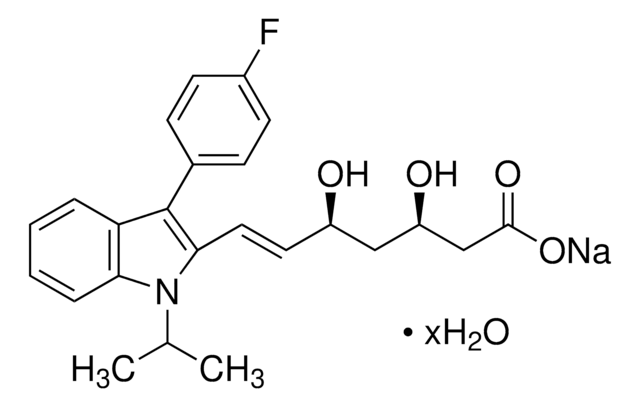
C24H25FNNaO4
CAS號碼:
分子量::
433.45
MDL號碼:
分類程式碼代碼:
41116107
PubChem物質ID:
NACRES:
NA.24
推薦產品
等級
pharmaceutical primary standard
API 家族
fluvastatin
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
形式
neat
SMILES 字串
[Na+].CC(C)n1c(\C=C\[C@H](O)C[C@H](O)CC([O-])=O)c(-c2ccc(F)cc2)c3ccccc13
InChI
1S/C24H26FNO4.Na/c1-15(2)26-21-6-4-3-5-20(21)24(16-7-9-17(25)10-8-16)22(26)12-11-18(27)13-19(28)14-23(29)30;/h3-12,15,18-19,27-28H,13-14H2,1-2H3,(H,29,30);/q;+1/p-1/b12-11+;/t18-,19-;/m0./s1
InChI 密鑰
ZGGHKIMDNBDHJB-RPQBTBOMSA-M
尋找類似的產品? 前往 產品比較指南
相關類別
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Fluvastatin for system suitability EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
相關產品
產品號碼
描述
訂價
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Ramakrishna Nirogi et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 907, 65-73 (2012-10-06)
Deferasirox is an iron chelating agent for the treatment of transfusional iron over load in patients with chronic anemia. These anemic patients require close monitoring of the deferasirox exposures for ensuring its therapeutic efficacy. Dried blood spot (DBS) sampling methodology
H R Xu et al.
Journal of biomedicine & biotechnology, 2012, 386230-386230 (2012-07-20)
The aim of this study was to evaluate the difference in pharmacokinetics and pharmacodynamics between extended-release (ER) fluvastatin tablet and its immediate-release (IR) capsule in Chinese healthy subjects. This was an open-label, single/multiple-dose, two-period, two-treatment, crossover, randomized trial with a
Masanori Atsukawa et al.
Journal of gastroenterology and hepatology, 28(1), 51-56 (2012-09-20)
Although the anti-hepatitis C virus (HCV) effect of statins in vitro and clinical efficacy of fluvastatin combined with Pegylated interferon (PEG-IFN)/ribavirin therapy for chronic hepatitis C (CHC) have been reported, the details of clinical presentation are largely unknown. We focused
C Kondo et al.
Journal of viral hepatitis, 19(9), 615-622 (2012-08-07)
Pegylated interferon (PEG-IFN)/ribavirin combination therapy is the standard-of-care (SOC) treatment for chronic hepatitis C patients infected with hepatitis C virus (HCV) genotype 1b and high viral load. The addition of fluvastatin to SOC treatment has been suggested to be effective
Allen B Williams et al.
Blood, 120(15), 3069-3079 (2012-08-29)
FLT3 is frequently mutated in acute myeloid leukemia (AML), but resistance has limited the benefit of tyrosine kinase inhibitors (TKI). We demonstrate that statins can impair FLT3 glycosylation, thus leading to loss of surface expression and induction of cell death
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務