推薦產品
等級
pharmaceutical primary standard
API 家族
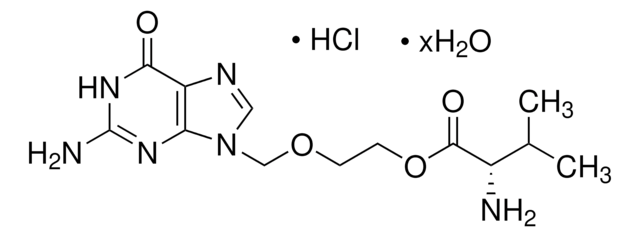
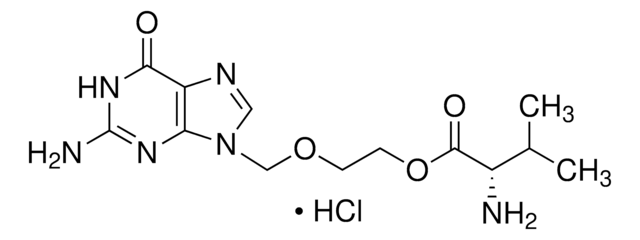
valacyclovir
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
2-8°C
SMILES 字串
Cl.CC(C)[C@H](N)C(=O)OCCOCn1cnc2C(=O)NC(N)=Nc12
InChI
1S/C13H20N6O4.ClH/c1-7(2)8(14)12(21)23-4-3-22-6-19-5-16-9-10(19)17-13(15)18-11(9)20;/h5,7-8H,3-4,6,14H2,1-2H3,(H3,15,17,18,20);1H/t8-;/m0./s1
InChI 密鑰
ZCDDBUOENGJMLV-QRPNPIFTSA-N
尋找類似的產品? 前往 產品比較指南
相關類別
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
Valacyclovir is a prodrug of acyclovir. It is an L-valyl ester and works against herpes simplex virus type and varicella zoster virus.
應用
This European Pharmacopoeia reference standard is intended for use only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
UV spectrophotometric method for the estimation of valacyclovir hcl in tablet dosage form.
Ganesh, M., et al.
Journal of Chemistry, 6.3, 814-818 (2009)
Selective and rapid liquid chromatography/negative-ion electrospray ionization mass spectrometry method for the quantification of valacyclovir and its metabolite in human plasma.
Kasiari M
Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences, 864(1-2), 78-86 (2008)
Shlomit Schaal et al.
JAMA ophthalmology, 132(7), 881-882 (2014-04-20)
Acute retinal necrosis (ARN) is an infectious retinitis primarily caused by the herpesviruses. Although the Epstein-Barr virus (EBV) has been implicated as a cause of ARN, to our knowledge, there has been no histopathologic documentation. We report the clinical history
Mary J Burton et al.
The American journal of the medical sciences, 348(6), 455-459 (2014-08-28)
We performed a pilot study examining the safety and tolerability of valacyclovir in veterans with herpes simplex virus type 2 and hepatitis C virus (HCV) coinfection. We performed a randomized double-blind, placebo-controlled, crossover clinical trial in U.S. veterans with genotype
Laurence Le Cleach et al.
The Cochrane database of systematic reviews, 8(8), CD009036-CD009036 (2014-08-05)
Genital herpes is caused by herpes simplex virus 1 (HSV-1) or 2 (HSV-2). Some infected people experience outbreaks of genital herpes, typically, characterized by vesicular and erosive localized painful genital lesions. To compare the effectiveness and safety of three oral
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務