全部照片(1)
About This Item
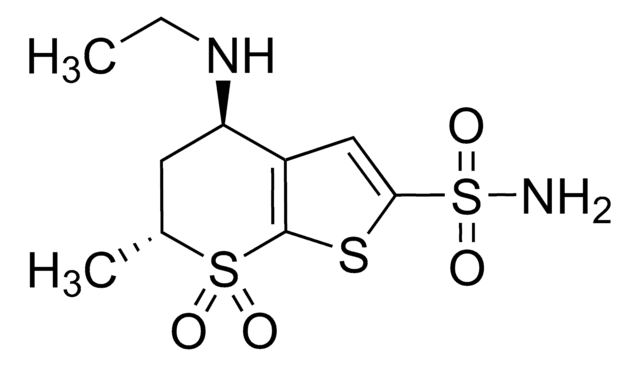
經驗公式(希爾表示法):
C10H16N2O4S3 · HCl
CAS號碼:
分子量::
360.90
Beilstein:
5896026
MDL號碼:
分類程式碼代碼:
41116107
PubChem物質ID:
NACRES:
NA.24
推薦產品
等級
pharmaceutical primary standard
API 家族
dorzolamide
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
形式
neat
SMILES 字串
Cl.CCN[C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O
InChI
1S/C10H16N2O4S3.ClH/c1-3-12-8-4-6(2)18(13,14)10-7(8)5-9(17-10)19(11,15)16;/h5-6,8,12H,3-4H2,1-2H3,(H2,11,15,16);1H/t6-,8-;/m0./s1
InChI 密鑰
OSRUSFPMRGDLAG-QMGYSKNISA-N
基因資訊
human ... CA2(760)
尋找類似的產品? 前往 產品比較指南
相關類別
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Dorzolamide for system suitability EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
相關產品
產品號碼
描述
訂價
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral - STOT RE 2
標靶器官
Central nervous system,Gastrointestinal tract,Bone,Blood,Bladder
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Yasuhiro Ikeda et al.
The British journal of ophthalmology, 97(9), 1187-1191 (2013-06-21)
To evaluate the therapeutic effect of continuous treatment with topical dorzolamide (a carbonic anhydrase inhibitor) for cystoid macular oedema (CME) associated with retinitis pigmentosa (RP). 18 eyes in 10 patients with CME secondary to RP were included. Baseline visual acuity
Yasuhiro Ikeda et al.
Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie, 250(6), 809-814 (2012-01-05)
Cystoid macular edema (CME) is one of the common complications of retinitis pigmentosa (RP), and is responsible for patient complications such as blurred and reduced visual acuity and for subsequent atrophic changes in the fovea. The objective of this work
Ikuyo Ohguro et al.
Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics, 28(4), 392-396 (2012-04-07)
The purpose of the present study is to evaluate the effects of a fixed combination of 0.5% timolol maleate (TM) and 1% dorzolamide hydrochloride (DZ) (FCTD(1%)) on optic nerve head (ONH) blood circulation. A drop of 0.5% TM, 1% DZ
Vikas Gulati et al.
Archives of ophthalmology (Chicago, Ill. : 1960), 130(6), 677-684 (2012-02-15)
To evaluate the interaction of intraocular pressure(IOP)–lowering medications with physiologic day and night changes in aqueous humor dynamics in participants with ocular hypertension. Thirty participants were enrolled in thisdouble-masked, randomized, crossover study. Each participant underwent aqueous humor dynamics measurements at
Mumin Hakan Eren et al.
Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics, 28(4), 381-386 (2012-02-11)
To compare the efficacy and safety of dorzolamide hydrochloride 2%/timolol maleate 0.5% fixed combination (DTFC) with latanoprost 0.005%/timolol maleate 0.5% fixed combination (LTFC) on diurnal intraocular pressure (IOP) in patients with primary open-angle glaucoma. Thirty-three primary open-angle glaucoma patients with
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務