推薦產品
等級
pharmaceutical primary standard
API 家族
midazolam
製造商/商標名
EDQM
藥物控制
regulated under CDSA - not available from Sigma-Aldrich Canada; psicótropo (Spain); Decreto Lei 15/93: Tabela IV (Portugal)
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
2-8°C
SMILES 字串
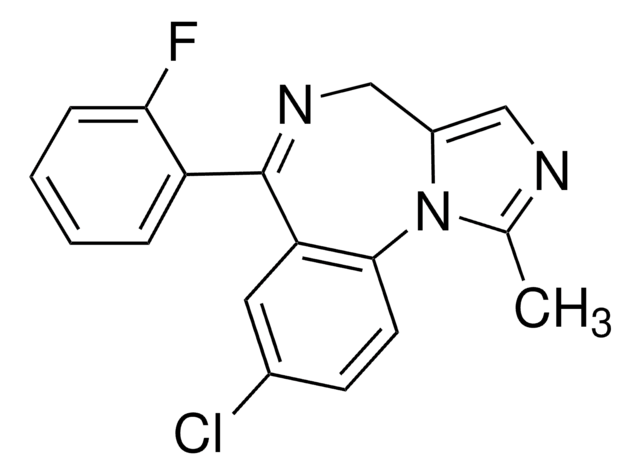
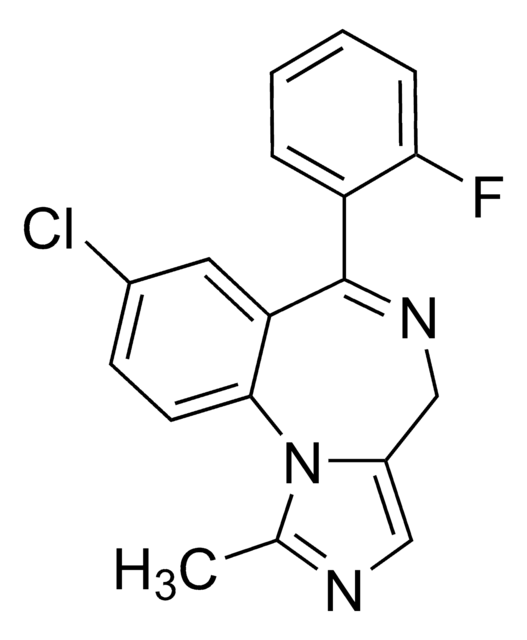
Fc1c(cccc1)C2=NCc3[n](c(nc3)C)c4c2cc(cc4)Cl
InChI
1S/C18H13ClFN3/c1-11-21-9-13-10-22-18(14-4-2-3-5-16(14)20)15-8-12(19)6-7-17(15)23(11)13/h2-9H,10H2,1H3
InChI 密鑰
DDLIGBOFAVUZHB-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Midazolam for system suitability EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Question 3 Ketamine or midazolam: does it matter which?
Elisabeth Jameson
Archives of disease in childhood, 96(1), 106-108 (2010-12-21)
Eugene Ng et al.
The Cochrane database of systematic reviews, 6(6), CD002052-CD002052 (2012-06-15)
Proper sedation for neonates undergoing uncomfortable procedures may reduce stress and avoid complications. Midazolam is a short-acting benzodiazepine that is increasingly used in neonatal intensive care units (NICU). However, its effectiveness as a sedative in neonates has not been systematically
Karly P Garnock-Jones
Paediatric drugs, 14(4), 251-261 (2012-06-19)
Oromucosal midazolam (Buccolam™) is a benzodiazepine approved for the treatment of pediatric patients with acute, prolonged, convulsive seizures. This article reviews the pharmacologic properties of oromucosal midazolam and its clinical efficacy and tolerability for the treatment of prolonged acute convulsive
P Jevon
British dental journal, 213(2), 81-82 (2012-07-28)
This article is published in response to a query from reader Sarah Clements, based at Golding House Dental Practice Ltd in Kent, regarding the BDJ paper entitled 'Updated guidance on medical emergencies and resuscitation in the dental practice' (BDJ 2012;
Eleonora L Swart et al.
Current drug metabolism, 13(6), 760-766 (2012-03-29)
A variety of developmental changes is of influence on the pharmacokinetics and pharmacodynamics of midazolam in neonatal and pediatric intensive care patients. However, dosing regimens in children are based upon rather empirical extrapolations from the dosing regimens in adults. Based
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務