全部照片(1)
About This Item
經驗公式(希爾表示法):
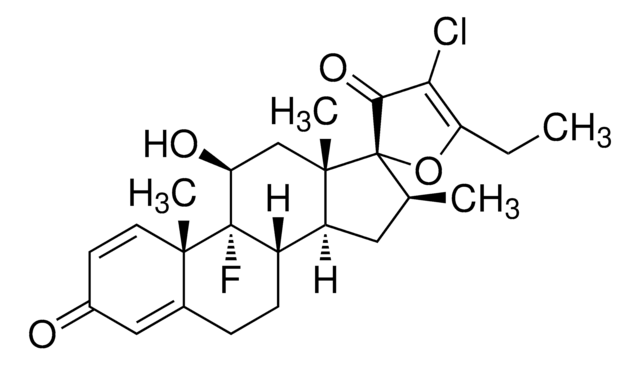
C25H32ClFO5
CAS號碼:
分子量::
466.97
MDL號碼:
分類程式碼代碼:
41116107
PubChem物質ID:
NACRES:
NA.24
推薦產品
等級
pharmaceutical primary standard
API 家族
clobetasol
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
2-8°C
SMILES 字串
CCC(=O)O[C@@]1([C@@H](C)C[C@H]2[C@@H]3CCC4=CC(=O)C=C[C@]4(C)[C@@]3(F)[C@@H](O)C[C@]12C)C(=O)CCl
InChI
1S/C25H32ClFO5/c1-5-21(31)32-25(20(30)13-26)14(2)10-18-17-7-6-15-11-16(28)8-9-22(15,3)24(17,27)19(29)12-23(18,25)4/h8-9,11,14,17-19,29H,5-7,10,12-13H2,1-4H3/t14-,17-,18-,19-,22-,23-,24-,25-/m0/s1
InChI 密鑰
CBGUOGMQLZIXBE-XGQKBEPLSA-N
基因資訊
human ... NR3C1(2908)
尋找類似的產品? 前往 產品比較指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Clobetasol propionate EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
相關產品
產品號碼
描述
訂價
訊號詞
Danger
危險聲明
危險分類
Aquatic Chronic 4 - Repr. 1B - STOT RE 2
標靶器官
Adrenal gland,Immune system
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
M Hofmann et al.
Archives of dermatological research, 305(3), 215-221 (2012-12-18)
Currently, there are no accurate and simple methods available to measure this risk of atrophy in patients treated with topical glucocorticosteroids. In the present clinical trial, we validated a new score (Dermaphot(®) score) to assess the atrophogenic potential of glucocorticosteroids.
Hetal K Patel et al.
Colloids and surfaces. B, Biointerfaces, 102, 86-94 (2012-09-25)
The aim of the present investigation was to evaluate microemulsion as a vehicle for dermal drug delivery and to develop microemulsion based gel (MBC) of clobetasol propionate (CP) for the effective treatment of vitiligo. D-Optimal mixture experimental design was adopted
Gionata Buggiani et al.
Dermatologic therapy, 25(5), 472-476 (2012-10-11)
Current vitiligo treatments are not always satisfactory for both patients and dermatologists. Recently, combination therapies have been introduced in order to obtain better results and reduce risks in the management of the disease. Novel efficacious products are needed to improve
Leon H Kircik et al.
Journal of drugs in dermatology : JDD, 12(3), 328-334 (2013-04-03)
Chronic hand dermatitis may have a significant detrimental effect on daily home-related and work-related activities, and quality of life (QOL). Clobetasol propionate foam, 0.05%, is indicated for the treatment of inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses in patients aged
Sidharth Sonthalia et al.
International journal of dermatology, 51(11), 1371-1378 (2012-10-17)
Oral lichen planus (OLP) is a common disease of the oral mucosa with worldwide distribution and overall prevalence of 0.5-2.2%. Its etiology remains unclear, although the role of autoimmunity is supported by its association with other autoimmune diseases and the
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務