推薦產品
等級
pharmaceutical primary standard
API 家族
propylene glycol
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
2-8°C
SMILES 字串
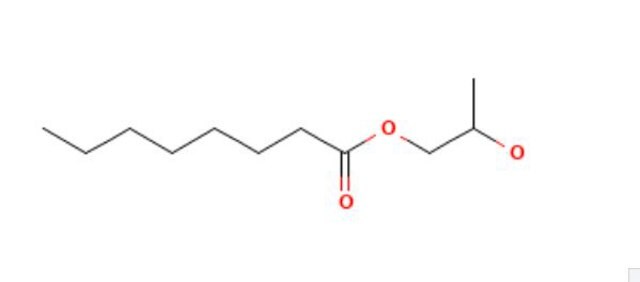
O(CC(O)C)C(=O)CCCCCCCCCCC
InChI
1S/C15H30O3/c1-3-4-5-6-7-8-9-10-11-12-15(17)18-13-14(2)16/h14,16H,3-13H2,1-2H3
InChI 密鑰
BHIZVZJETFVJMJ-UHFFFAOYSA-N
相關類別
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Propylene glycol monolaurate EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
相關產品
產品號碼
描述
訂價
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
Rana Abu-Huwaij et al.
Drug development and industrial pharmacy, 33(4), 437-448 (2007-05-25)
The aim of this study was to develop a controlled release buccal mucoadhesive delivery system for systemic delivery of lidocaine hydrochloride as a model drug. In vitro release and buccal permeation as well as in vivo permeation of LDHCL patches
Sang-Chul Shin et al.
Archives of pharmacal research, 29(10), 928-933 (2006-11-24)
Percutaneous delivery of NSAIDs has advantages of avoiding hepatic first pass effect and delivering the drug for extended period of time at a sustained, concentrated level at the inflammation site that mainly acts at the joint and the related regions.
B R Jasti et al.
The journal of investigative dermatology. Symposium proceedings, 3(2), 128-130 (1998-09-12)
Excipients are often used in transdermal formulations to overcome the formidable barrier offered by the epidermis in order to achieve the target flux. In this study we describe the use of frequency-domain fluorescence spectroscopy to characterize the effect of two
Jian Meng et al.
Drug development and industrial pharmacy, 33(9), 927-931 (2007-09-25)
Self-microemulsifying drug delivery systems (SMEDDS) are useful to improve the bioavailability of poorly water-soluble drugs by increasing their apparent solubility through solubilization. However, very few studies, to date, have systematically examined the level of drug apparent solubility in o/w microemulsion
Archita Patel et al.
Current drug delivery, 12(6), 745-760 (2015-03-04)
The solid-self nanoemulsifying drug delivery system (S-SNEDDS) of Amiodarone hydrochloride (AH) was prepared and evaluated. AH exhibits poor aqueous solubility (0.3-0.5 mg/ml) and therefore variable oral bioavailability. Capmul MCM, Cremophor RH-40 and Propylene glycol were identified as oil, surfactant and
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務