Y0000221
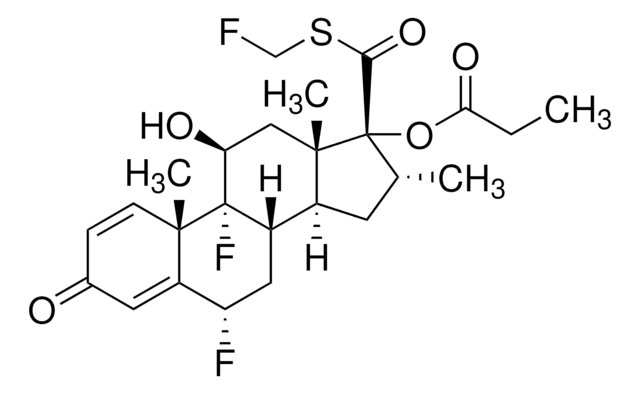
Fluticasone impurity D
European Pharmacopoeia (EP) Reference Standard
同義詞:
6α,9-Difluoro-17-[(methylsulfanyl)carbonyl]-11β-hydroxy-16α-methyl-3-oxoandrosta-1,4-dien-17α-yle propanoate, S-Methyl 6α, 9α-difluoro-11β-hydroxy-16α-methyl-3-oxo-17α-propionyloxyandrosta-1,4-diene-17β-carbothioate, Ticabesone propionate
登入查看組織和合約定價
全部照片(1)
About This Item
推薦產品
等級
pharmaceutical primary standard
API 家族
fluticasone
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
2-8°C
InChI
1S/C25H32F2O5S/c1-6-20(30)32-25(21(31)33-5)13(2)9-15-16-11-18(26)17-10-14(28)7-8-22(17,3)24(16,27)19(29)12-23(15,25)4/h7-8,10,13,15-16,18-19,29H,6,9,11-12H2,1-5H3/t13-,15?,16?,18+,19+,22?,23+,24+,25+/m1/s1
InChI 密鑰
DRXCUKXWTNOXTD-XTVONRPDSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Fluticasone impurity D EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
PLEASE NOTE: TEMPORARILY UNAVAILABLE DUE TO RESTRICTED QUANTITY
相關產品
產品號碼
描述
訂價
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務