推薦產品
等級
pharmaceutical primary standard
API 家族
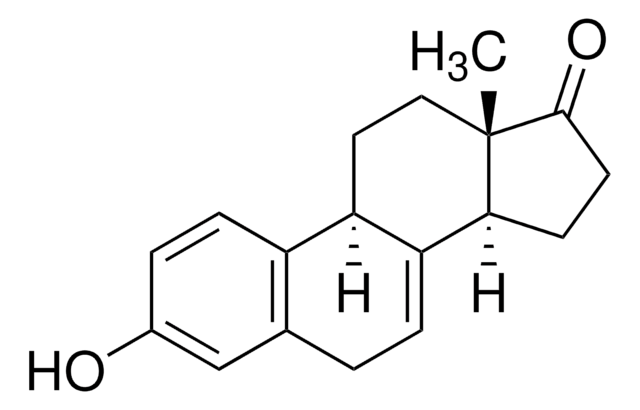
equilin
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
−20°C
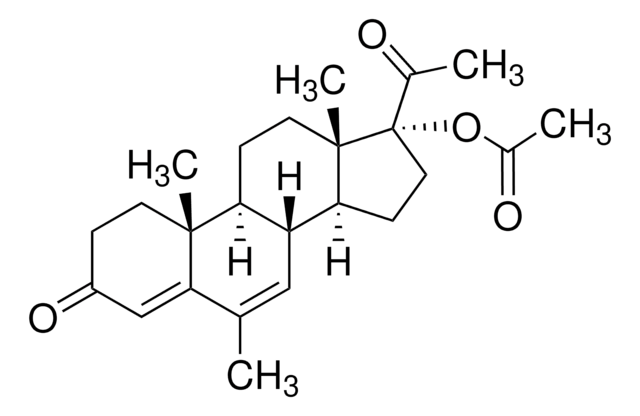
SMILES 字串
OC1C2(C(CC1)C3=CCc4c(ccc(c4)O)C3CC2)C
InChI
1S/C18H22O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3-5,10,14,16-17,19-20H,2,6-9H2,1H3
InChI 密鑰
NLLMJANWPUQQTA-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
17α-Dihydroequilin EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
B R Bhavnani et al.
Journal of the Society for Gynecologic Investigation, 7(3), 175-183 (2000-06-24)
To compare the pharmacokinetics and relative bioavailabilities of key estrogen components of Premarin (Wyeth-Ayerst, Canada) with those of a generic conjugated estrogen preparation, C.E.S. (synthetic mixture of estrogens; ICN, Montreal, Canada) in healthy postmenopausal women. We conducted a randomized, single-dose
S B Lima et al.
Journal of chromatographic science, 39(9), 385-387 (2001-09-22)
Equine unsaturated estrogens are the main components of brand formulations indicated for hormonal replacement therapy in both hypogonadic and postmenopausal women. These hormones are produced by the fetoplacental unit during equine gestation. A method is described for the quantitative determination
B R Bhavnani et al.
Steroids, 56(4), 201-210 (1991-04-01)
The present investigation was undertaken to compare the binding affinities (Ka) of the ring B unsaturated equine estrogens (equilin [Eq], equilenin [Eqn], 17 beta-dihydroequilin [17 beta-Eq], 17 beta-dihydroequilenin [17 beta-Eqn], 17 alpha-dihydroequilin [17 alpha-Eq], and17 alpha-dihydroequilenin [17 alpha-Eqn]) and the
B R Bhavnani et al.
Steroids, 59(6), 389-394 (1994-06-01)
The metabolism of 17 beta-dihydroequilin and 17 beta-dihydroequilin sulfate was investigated after intravenous administration of [3H] 17 beta-dihydroequilin and [3H] 17 beta-dihydroequilin sulfate to postmenopausal women. Urine was collected for 3 days and 46.2 +/- 10.5% and 54.5 +/- 8.7%
B R Bhavnani et al.
The Journal of clinical endocrinology and metabolism, 78(1), 197-204 (1994-01-01)
The MCRs of 17 beta-dihydroequilin sulfate and 17 beta-dihydroequilin were determined in normal postmenopausal women by single iv injection of either 17 beta-[3H]dihydroequilin sulfate ([3H]17 beta-EqS) or 17 beta-[3H]dihydroequilin ([3H]17 beta-Eq). After the administration of [3H]17 beta-EqS, blood was drawn
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務