Y0000027
Sumatriptan for system suitability
European Pharmacopoeia (EP) Reference Standard
同義詞:
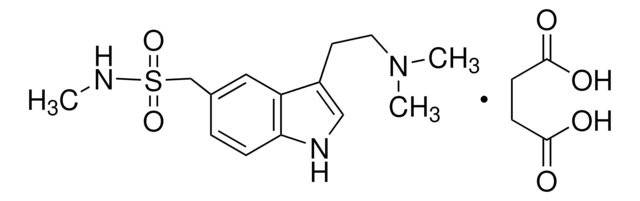
Sumatriptan succinate, 3-[2-(Dimethylamino)ethyl]-N-methyl-1H-indole-5-methanesulfonamide succinate, GR-43175
登入查看組織和合約定價
全部照片(1)
About This Item
經驗公式(希爾表示法):
C14H21N3O2S · C4H6O4
CAS號碼:
分子量::
413.49
MDL號碼:
分類程式碼代碼:
41116107
PubChem物質ID:
NACRES:
NA.24
推薦產品
等級
pharmaceutical primary standard
API 家族
sumatriptan
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
−20°C
SMILES 字串
OC(=O)CCC(O)=O.CNS(=O)(=O)Cc1ccc2[nH]cc(CCN(C)C)c2c1
InChI
1S/C14H21N3O2S.C4H6O4/c1-15-20(18,19)10-11-4-5-14-13(8-11)12(9-16-14)6-7-17(2)3;5-3(6)1-2-4(7)8/h4-5,8-9,15-16H,6-7,10H2,1-3H3;1-2H2,(H,5,6)(H,7,8)
InChI 密鑰
PORMUFZNYQJOEI-UHFFFAOYSA-N
基因資訊
human ... HTR1B(3351) , HTR1D(3352)
尋找類似的產品? 前往 產品比較指南
相關類別
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Sumatriptan for system suitability EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
生化/生理作用
Sumatriptan succinate is a 5-HT1 serotonin receptor agonist.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
相關產品
產品號碼
描述
訂價
訊號詞
Warning
危險聲明
危險分類
Aquatic Chronic 3 - Repr. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Christian Asseburg et al.
International journal of technology assessment in health care, 28(4), 382-389 (2012-09-28)
The cost-effectiveness of triptans in the treatment of migraine has not been assessed since generic sumatriptan entered the Finnish market in 2008. Using systematic review and mixed treatment comparison, the effectiveness of triptans was estimated with regard to 2-hour response
Daxesh P Patel et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 902, 122-131 (2012-07-24)
An ultra performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) method has been developed for the simultaneous determination of sumatriptan and naproxen in human plasma using naratriptan and indomethacin as the internal standards (ISs). The plasma samples were prepared by solid phase
Benjamin W Friedman et al.
Academic emergency medicine : official journal of the Society for Academic Emergency Medicine, 19(10), 1151-1157 (2012-09-22)
Patients who use an emergency department (ED) for acute migraine headaches have higher migraine disability scores, lower socioeconomic status, and are unlikely to have used a migraine-specific medication prior to presentation to the ED. The objective was to determine if
Toshihiko Tomita et al.
Journal of gastroenterology and hepatology, 28(1), 106-111 (2012-09-20)
Scintigraphy is a useful noninvasive technique for assessment of gastric motility, especially emptying, but there is little knowledge of use of the technique to assess gastric accommodation. Therefore, to clarify the usefulness of scintigraphy as a technique for assessing gastric
Maria Luisa González-Rodríguez et al.
Journal of pharmaceutical sciences, 101(10), 3845-3863 (2012-07-19)
Niosomes formulated from different nonionic surfactants (Span® 60, Brij® 72, Span® 80, or Eumulgin® B 2) with cholesterol (CH) molar ratios of 3:1 or 4:1 with respect to surfactant were prepared with different sumatriptan amount (10 and 15 mg) and
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務