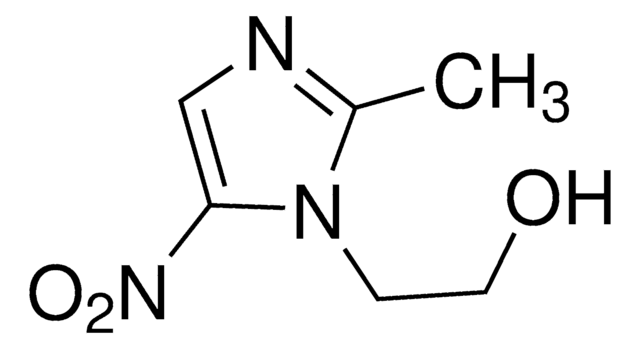
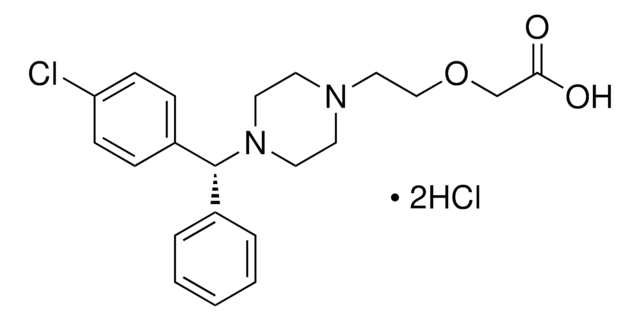
推薦產品
等級
pharmaceutical secondary standard
reference material
品質等級
agency
traceable to USP 1269254
包裝
pkg of 50 mg
應用
pharmaceutical small molecule
InChI
1S/C10H14N8S4/c11-7(12)17-9-15-5(1-19-9)3-21-22-4-6-2-20-10(16-6)18-8(13)14/h1-2H,3-4H2,(H4,11,12,15,17)(H4,13,14,16,18)
InChI 密鑰
ZWHJVLVEEDAPHN-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
相關類別
一般說明
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards
應用
These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
分析報告
These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務