推薦產品
等級
certified reference material
pharmaceutical secondary standard
品質等級
agency
traceable to USP 1381119
API 家族
menaquinone
形狀
solid
CofA
current certificate can be downloaded
包裝
pkg of 3x75 mg
應用
pharmaceutical small molecule
儲存溫度
-10 to -25°C
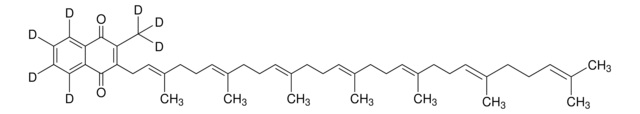
InChI
1S/C46H64O2/c1-34(2)18-12-19-35(3)20-13-21-36(4)22-14-23-37(5)24-15-25-38(6)26-16-27-39(7)28-17-29-40(8)32-33-42-41(9)45(47)43-30-10-11-31-44(43)46(42)48/h10-11,18,20,22,24,26,28,30-32H,12-17,19,21,23,25,27,29,33H2,1-9H3/b35-20+,36-22+,37-24+,38-26+,39-28+,40-32+
InChI 密鑰
RAKQPZMEYJZGPI-LJWNYQGCSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
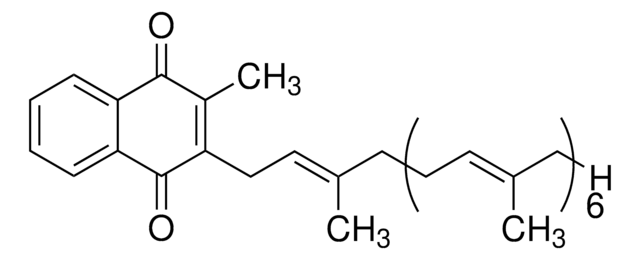
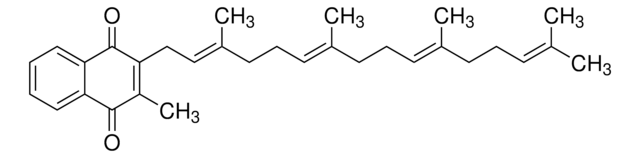
Menaquinone -7 is a naphthoquinone derivative and one of the most active sub-type of vitamin K2, with 7 referring to the number of isoprene units in its structure. It is naturally found in fermented dairy products and is considered a nutrient, thereby being found as a component of various nutritional supplements.
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Menaquinone -7 is a naphthoquinone derivative and one of the most active sub-type of vitamin K2, with 7 referring to the number of isoprene units in its structure. It is naturally found in fermented dairy products and is considered a nutrient, thereby being found as a component of various nutritional supplements.
應用
This pharmaceutical secondary standard can also be used as follows:
- Ultra high-performance liquid chromatography (UHPLC) based determination of menaquinone-7 and vitamin D3 in their combined tablet formulation
- Development of a thin-layer chromatographic-densitometric technique for the quantitative analysis of vitamins K and D3 in pharmaceutical tablet formulations and dietary supplements
- Simultaneous determination of vitamin D3 and K2 (Menaquinone-4 and Menaquinone-7) in dietary supplements by ultra-high-pressure liquid chromatography (UHPLC)
- Impurity profiling of menaquinone-7 in its active pharmaceutical ingredient (API) samples using HPLC coupled to UV detector
- Analysis of menaquinone-4 and menaquinone-7 by HPLC in combination with tandem mass spectrometry (MS/MS) in human milk samples
分析報告
These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available.
腳註
To see an example of a Certificate of Analysis for this material enter LRAC4030 in the Documents slot below. This is an example certificate only and may not be the lot that you receive.
推薦產品
Find a digital Reference Material for this product available on our online platform ChemisTwin® for NMR. You can use this digital equivalent on ChemisTwin® for your sample identity confirmation and compound quantification (with digital external standard). An NMR spectrum of this substance can be viewed and an online comparison against your sample can be performed with a few mouseclicks. Learn more here and start your free trial.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
Łukasz Jedynak et al.
Journal of pharmaceutical and biomedical analysis, 135, 116-125 (2016-12-27)
An HPLC method with UV detection and separation with the use of a C30 reversed phase analytical column for the determination of chemical purity and assay of menaquinone-7 (MK7) in one chromatographic run was developed. The method is superior to
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務