推薦產品
等級
certified reference material
pharmaceutical secondary standard
品質等級
agency
traceable to Ph. Eur. D0740000
traceable to USP 1181007
API 家族
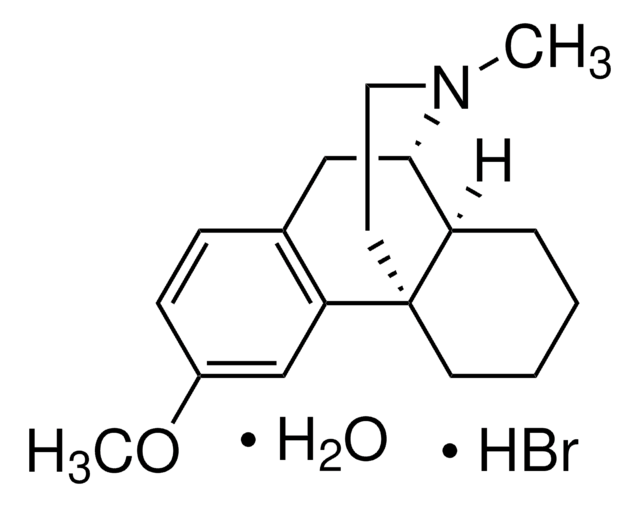
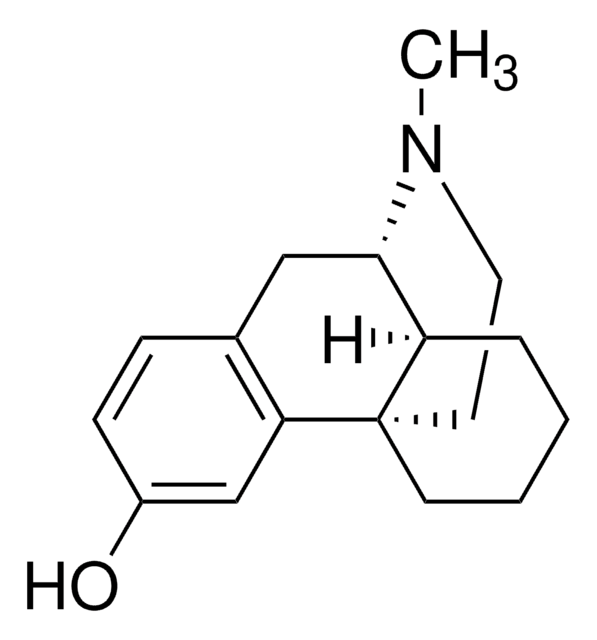
dextromethorphan
CofA
current certificate can be downloaded
技術
HPLC: suitable
gas chromatography (GC): suitable
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
2-30°C
SMILES 字串
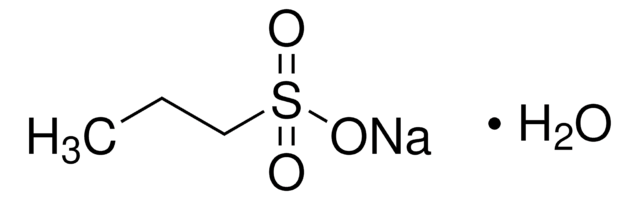
Br[H].[H]O[H].[H][C@]12CCCC[C@]13CCN(C)[C@H]2Cc4ccc(OC)cc34
InChI
1S/C18H25NO.BrH.H2O/c1-19-10-9-18-8-4-3-5-15(18)17(19)11-13-6-7-14(20-2)12-16(13)18;;/h6-7,12,15,17H,3-5,8-11H2,1-2H3;1H;1H2/t15-,17+,18+;;/m1../s1
InChI 密鑰
STTADZBLEUMJRG-IKNOHUQMSA-N
基因資訊
human ... GRIN2A(2903) , SIGMAR1(10280)
尋找類似的產品? 前往 產品比較指南
一般說明
應用
分析報告
其他說明
腳註
相關產品
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral - Aquatic Chronic 2
儲存類別代碼
6.1D - Non-combustible acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
條款
Silica gel G 254 plates are suitable for analysis of Dextromethorphan following the European pharmacopeia monograph.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務