推薦產品
等級
pharmaceutical primary standard
API 家族
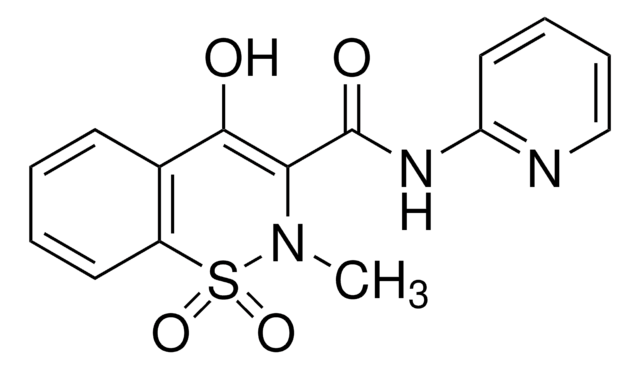
piroxicam
製造商/商標名
EDQM
技術
HPLC: suitable
gas chromatography (GC): suitable
應用
pharmaceutical (small molecule)
形式
neat
SMILES 字串
CN1C(C(=O)Nc2ccccn2)=C(O)c3ccccc3S1(=O)=O
InChI
1S/C15H13N3O4S/c1-18-13(15(20)17-12-8-4-5-9-16-12)14(19)10-6-2-3-7-11(10)23(18,21)22/h2-9,19H,1H3,(H,16,17,20)
InChI 密鑰
QYSPLQLAKJAUJT-UHFFFAOYSA-N
基因資訊
human ... PTGS1(5742) , PTGS2(5743)
尋找類似的產品? 前往 產品比較指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Piroxicam EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
生化/生理作用
环加氧酶抑制剂。
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
相關產品
產品號碼
描述
訂價
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral - STOT RE 2 Oral
標靶器官
Gastrointestinal tract
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Wanwadee Luksurapan et al.
Archives of physical medicine and rehabilitation, 94(2), 250-255 (2012-10-16)
To compare the effects of phonophoresis of piroxicam (PhP) and ultrasound therapy (UT) in patients with mild to moderate, symptomatic knee osteoarthritis (OA). A randomized, double-blind, controlled trial. Department of rehabilitation medicine, university hospital. Patients with knee OA (N=46; mean
Andres Lust et al.
European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences, 48(1-2), 47-54 (2012-10-23)
The aim of this study was to gain understanding about the effects of different solid-state forms of a poorly water-soluble piroxicam on drug dissolution and oral bioavailability in rats. Three different solid-state forms of piroxicam were studied: anhydrate I (AH)
Jian X Wu et al.
Journal of pharmaceutical sciences, 102(3), 904-914 (2012-12-06)
Several factors with complex interactions influence the physical stability of solid dispersions, thus highlighting the need for efficient experimental design together with robust and simple multivariate model. Design of Experiments together with ANalysis Of VAriance (ANOVA) model is one of
Xin-Yue Song et al.
Talanta, 100, 153-161 (2012-11-13)
A novel design of carbon nanotubes reinforced hollow fiber solid/liquid phase microextraction (CNTs-HF-SLPME) was developed to determine piroxicam and diclofenac in different real water samples. Functionalized multi-walled carbon nanotubes (MWCNTs) were held in the pores of hollow fiber with sol-gel
Michael A Kron et al.
Clinical and vaccine immunology : CVI, 20(2), 276-281 (2012-12-21)
The therapeutic effects of a controlled parasitic nematode infection on the course of inflammatory bowel disease (IBD) have been demonstrated in both animal and human models. However, the inability of individual well-characterized nematode proteins to recreate these beneficial effects has
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務