推薦產品
等級
pharmaceutical primary standard
API 家族
pimozide
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
2-8°C
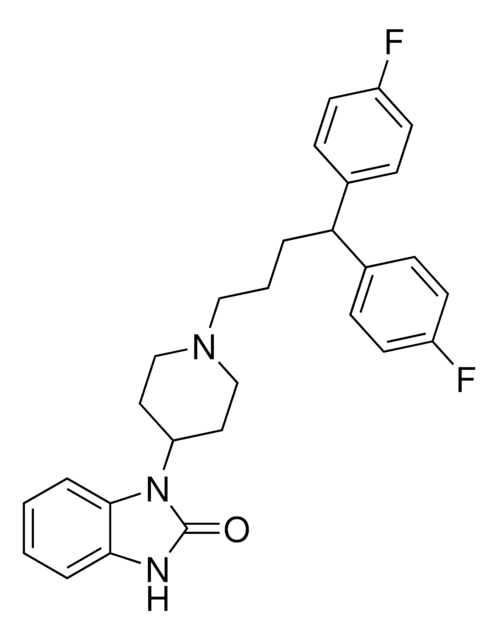
SMILES 字串
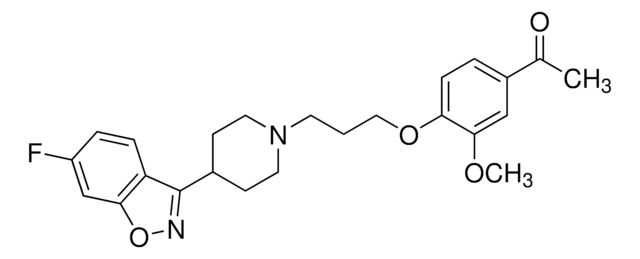
Fc1ccc(cc1)C(CCCN2CCC(CC2)N3C(=O)Nc4ccccc34)c5ccc(F)cc5
InChI
1S/C28H29F2N3O/c29-22-11-7-20(8-12-22)25(21-9-13-23(30)14-10-21)4-3-17-32-18-15-24(16-19-32)33-27-6-2-1-5-26(27)31-28(33)34/h1-2,5-14,24-25H,3-4,15-19H2,(H,31,34)
InChI 密鑰
YVUQSNJEYSNKRX-UHFFFAOYSA-N
基因資訊
human ... DRD1(1812) , DRD2(1813) , DRD3(1814) , DRD4(1815) , DRD5(1816) , HTR2A(3356)
尋找類似的產品? 前往 產品比較指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Pimozide EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Cynthia R Lorenzo et al.
American journal of clinical dermatology, 5(5), 339-349 (2004-11-24)
Pimozide is an antipsychotic drug of the diphenylbutylpiperidine class. In the US, it is FDA-approved only as a backup treatment for Gilles de la Tourette syndrome, although it has been used in other countries for many years as a treatment
Tamara Pringsheim et al.
The Cochrane database of systematic reviews, (2)(2), CD006996-CD006996 (2009-04-17)
Neuroleptic drugs with potent D-2 receptor blocking properties have been the traditional treatment for tics caused by Tourette Syndrome. Pimozide is the most studied of these. Use of these medications is declining because of concerns about side effects, and new
Meghana Mothi et al.
The Cochrane database of systematic reviews, 11(11), CD001949-CD001949 (2013-11-07)
Pimozide, formulated in the 1960s, continues to be marketed for the care of people with schizophrenia or related psychoses such as delusional disorder. It has been associated with cardiotoxicity and sudden unexplained death. Electrocardiogram monitoring is now required before and
C L Colvin et al.
Drug intelligence & clinical pharmacy, 19(6), 421-424 (1985-06-01)
The orphan drug pimozide was recently approved for marketing in the U.S. for the treatment of Tourette's syndrome (TS). TS is characterized by recurrent, involuntary motor movements and vocal tics, and is believed to be due to neurochemical dysfunction. Pimozide's
J Rathbone et al.
The Cochrane database of systematic reviews, (3)(3), CD001949-CD001949 (2007-07-20)
Pimozide, formulated in the 1960s, continues to be marketed for the care of people with schizophrenia or related psychoses such as delusional disorder. It has been associated with cardiotoxicity and sudden unexplained deaths. Electrocardiogram monitoring is now required before and
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務