推薦產品
等級
pharmaceutical primary standard
API 家族
neostigmine
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
形式
neat
SMILES 字串
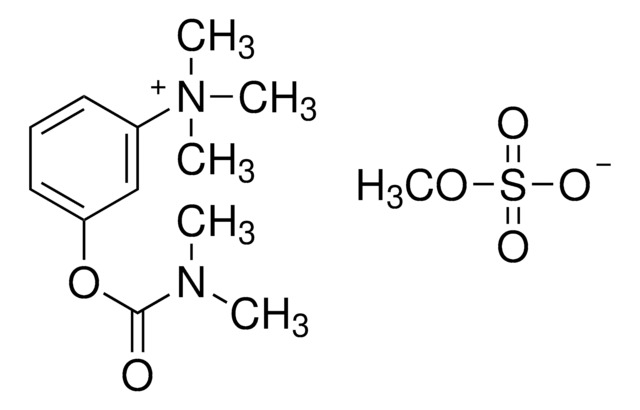
COS([O-])(=O)=O.CN(C)C(=O)Oc1cccc(c1)[N+](C)(C)C
InChI
1S/C12H19N2O2.CH4O4S/c1-13(2)12(15)16-11-8-6-7-10(9-11)14(3,4)5;1-5-6(2,3)4/h6-9H,1-5H3;1H3,(H,2,3,4)/q+1;/p-1
InChI 密鑰
OSZNNLWOYWAHSS-UHFFFAOYSA-M
基因資訊
human ... ACHE(43)
尋找類似的產品? 前往 產品比較指南
相關類別
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Neostigmine methyl sulfate EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
生化/生理作用
类似于毒扁豆碱的乙酰胆碱酯酶的可逆抑制剂,但是不能穿过血脑屏障。
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
訊號詞
Danger
危險分類
Acute Tox. 2 Oral - Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Jakub Fichna et al.
Pharmacological reports : PR, 64(5), 1146-1154 (2012-12-15)
Animal models of visceral pain have gained much attention as an important tool to elucidate the possible mechanisms underlying functional gastrointestinal (GI) disorders. Here we report the development of a new, minimally invasive behavioral model of abdominal pain induced by
Christian Lyngsaa Lang et al.
Ugeskrift for laeger, 175(16), 1120-1121 (2013-05-09)
The case report describes a 37-year-old woman who was diagnosed with Ogilvie's syndrome after caesarean section. Conservative treatment was initiated with minimal effect, and the patient was subsequently treated with IV neostigmine. A computed tomography of the abdomen revealed enlarged
Martina Grosse-Sundrup et al.
BMJ (Clinical research ed.), 345, e6329-e6329 (2012-10-19)
To determine whether use of intermediate acting neuromuscular blocking agents during general anesthesia increases the incidence of postoperative respiratory complications. Prospective, propensity score matched cohort study. General teaching hospital in Boston, Massachusetts, United States, 2006-10. 18,579 surgical patients who received
Oyun Khookhor et al.
Neuro endocrinology letters, 34(1), 58-61 (2013-03-26)
We previously demonstrated that the direct microinjection of cholinesterase inhibitor (neostigmine) into the hippocampus in rats activated the hypothalamo-pituitary -adrenal axis and increased the level of norepinephrine in the plasma. In the current study we tried to measure the effects
Failure of prefilled thiopental to induce anaesthesia.
P J Stewart et al.
Anaesthesia, 68(3), 308-308 (2013-02-07)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務