推薦產品
等級
pharmaceutical primary standard
API 家族
mepivacaine
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
形式
neat
SMILES 字串
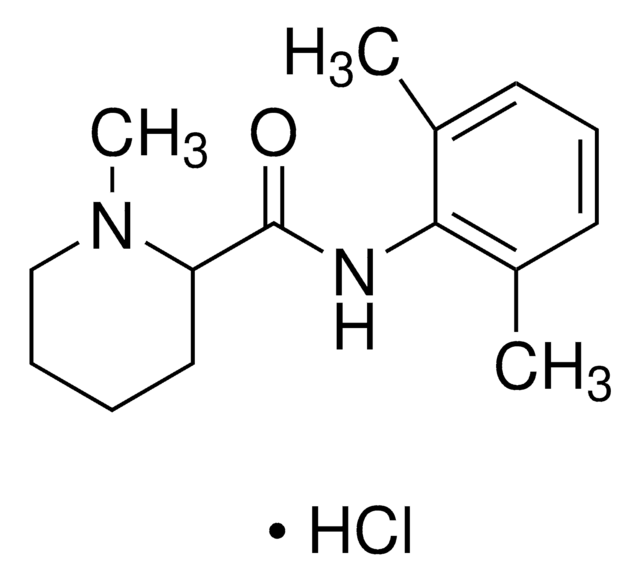
O=C(NC1=C(C)C=CC=C1C)C2NCCCC2
InChI
1S/C14H20N2O/c1-10-6-5-7-11(2)13(10)16-14(17)12-8-3-4-9-15-12/h5-7,12,15H,3-4,8-9H2,1-2H3,(H,16,17)
InChI 密鑰
SILRCGDPZGQJOQ-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
相關類別
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Mepivacaine impurity B EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
相關產品
產品號碼
描述
訂價
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 1
閃點(°F)
Not applicable
閃點(°C)
Not applicable
M Gantenbein et al.
Drug metabolism and disposition: the biological fate of chemicals, 28(4), 383-385 (2000-03-22)
Bupivacaine is used to provide prolonged anesthesia and postoperative analgesia. The human cytochrome P450 (CYP) involved in bupivacaine degradation into pipecolylxylidine (PPX), its major metabolite, has, to our knowledge, never been described. Microsome samples were prepared from six human livers
Sofia Berggren et al.
The Journal of pharmacy and pharmacology, 55(7), 963-972 (2003-08-09)
The major aim of this study was to investigate the CYP3A4 metabolism and polarized transport of ropivacaine and its metabolite 2',6'-pipecoloxylidide (PPX) in tissue specimens from the human small and large intestine. Ropivacaine has been shown to be effective in
S Reif et al.
Journal of chromatography. B, Biomedical sciences and applications, 719(1-2), 239-244 (1998-12-30)
A sensitive HPLC method has been developed for the determination of ropivacaine, 3-hydroxy-ropivacaine, 4-hydroxy-ropivacaine and 2',6'-pipecoloxylidide in plasma. The procedure involved extraction from plasma with a mixture of n-heptane-ethyl acetate and a back-extraction into an acidified aqueous solution. The chromatography
J Butterworth et al.
Anesthesia and analgesia, 85(2), 336-342 (1997-08-01)
Local anesthetics inhibit binding of ligands to beta2-adrenergic receptors (beta2ARs), and, as a consequence, inhibit intracellular cAMP production. We hypothesized that among homologous local anesthetics, their avidity at inhibiting binding of tritiated dihydroalprenolol (3H-DHA) to beta2ARs would increase with increasing
P J Pere et al.
British journal of anaesthesia, 106(4), 512-521 (2011-02-11)
As ropivacaine and its metabolites are excreted by the kidneys, we studied their disposition in subjects with renal dysfunction. Twenty patients with moderate or severe renal insufficiency and 10 healthy volunteers received ropivacaine 1 mg kg(-1) i.v. over 30 min.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務