推薦產品
等級
pharmaceutical primary standard
API 家族
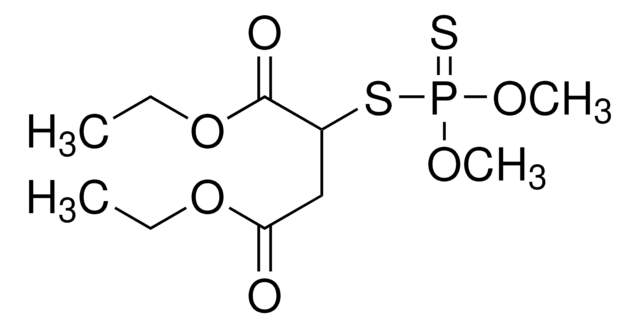
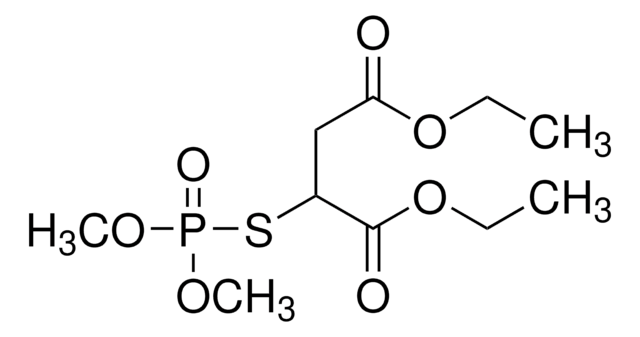
malathion
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
−20°C
InChI
1S/C10H19O6PS2/c1-5-15-9(11)7-8(10(12)16-6-2)19-17(13,14-3)18-4/h8H,5-7H2,1-4H3
InChI 密鑰
LPQDGVLVYVULMX-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Malathion impurity A EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral
儲存類別代碼
6.1C - Combustible, acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
141.8 °F
閃點(°C)
61 °C
J Błasiak et al.
Mutation research, 445(2), 275-283 (1999-11-27)
Malathion [S-(1,2-dicarboethoxyethyl)O,O-dimethyl phosphorodithioate] is a commonly used organophosphorus insecticide reported to be genotoxic both in vivo and in vitro, but the reports are conflicting. In order to elucidate the genotoxic potency of the main compounds present in commercial preparations of
J A Doorn et al.
Chemical research in toxicology, 14(7), 807-813 (2001-07-17)
Previous work has shown that acetylcholinesterase (AChE), a member of the alpha/beta-hydrolase superfamily, is stereoselectively inhibited by the four stereoisomers of isomalathion. Recent kinetic and mass spectral data demonstrated that a difference in mechanism of inactivation exists for AChE treated
S Xiong et al.
Journal of toxicology and environmental health, 51(2), 159-175 (1997-06-06)
In the present study, the effects of malathion and malathion derivatives on histamine and beta-hexosaminidase release by RBL-1 cells, rat peritoneal mast cells (RPMC), and human peripheral blood basophils (HPBB) and cutaneous mast calls were examined. One hour of incubation
Jonathan A Doorn et al.
Chemical research in toxicology, 16(8), 958-965 (2003-08-20)
The present study was undertaken to test the hypothesis that acetylcholinesterase (AChE) inhibition by isomalathion stereoisomers proceeds with different primary leaving groups for (1R)- and (1S)-isomers. Consistent with results obtained with enzyme from other species, AChE from Torpedo californica (TcAChE)
Franca M Buratti et al.
Journal of biochemical and molecular toxicology, 19(6), 406-414 (2006-01-20)
The organophosphorothioate (OPT) pesticide malathion (MAL) in mammals is readily hydrolyzed by mammalian carboxylesterases (CE). The reaction competes with the CYP-catalyzed formation of malaoxon (MOX), the toxic metabolite. Alterations or individual variations in CE activity may result in increased MOX
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務