推薦產品
等級
pharmaceutical primary standard
API 家族
fluphenazine
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
形式
neat
SMILES 字串
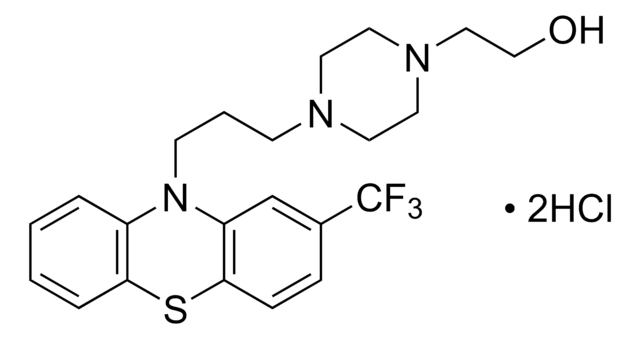
FC(F)(F)c1cc2c(cc1)Sc3c(cccc3)N2CCCN4CCN(CC4)CCOC(=O)CCCCCCCCC
InChI
1S/C32H44F3N3O2S/c1-2-3-4-5-6-7-8-14-31(39)40-24-23-37-21-19-36(20-22-37)17-11-18-38-27-12-9-10-13-29(27)41-30-16-15-26(25-28(30)38)32(33,34)35/h9-10,12-13,15-16,25H,2-8,11,14,17-24H2,1H3
InChI 密鑰
VIQCGTZFEYDQMR-UHFFFAOYSA-N
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Fluphenazine decanoate EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
相關產品
產品號碼
描述
訂價
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 2 Oral - Repr. 2
儲存類別代碼
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Janice Charles et al.
Australian family physician, 35(3), 88-89 (2006-03-10)
The BEACH program, a continuous national study of general practice activity in Australia, gives us an overview of consultations involving the management of psychoses. In this analysis we have included schizophrenia, affective disorders/bipolar, organic psychoses, and senile psychoses, with undefined
Rajaprabhakaran Rajarethinam et al.
The world journal of biological psychiatry : the official journal of the World Federation of Societies of Biological Psychiatry, 10(4 Pt 2), 416-419 (2009-06-06)
Aripiprazole, a partial dopamine agonist has been reported to help reduce symptoms of tardive dyskinesia (TD). In a prospective, open label study of a series of cases, we examined the effectiveness of aripiprazole in reducing TD symptoms. Six clinically stable
Mis-deca-n identity?
Elizabeth A S Giugni et al.
The Medical journal of Australia, 187(6), 370-370 (2007-09-19)
Zana Stanković et al.
Psychiatria Danubina, 20(1), 42-52 (2008-04-01)
To compare patient's attitudes, demographic, clinical characteristics, psychopathology, insight and type of antipsychotic therapy in compliant and non-compliant outpatients with schizophrenia; to explore correlations between patient's attitudes and related variables. A sample of 44 outpatients of both genders (> 60
Martin Turner et al.
International clinical psychopharmacology, 19(4), 241-249 (2004-06-18)
Long-acting injectable risperidone was assessed in schizophrenia patients who were symptomatically stable on conventional depot antipsychotics and who were then switched to long-acting risperidone. Participants in this open-label, multicentre, 12-week trial had received flupenthixol decanoate, fluphenazine decanoate, haloperidol decanoate, or
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務