推薦產品
等級
pharmaceutical primary standard
API 家族
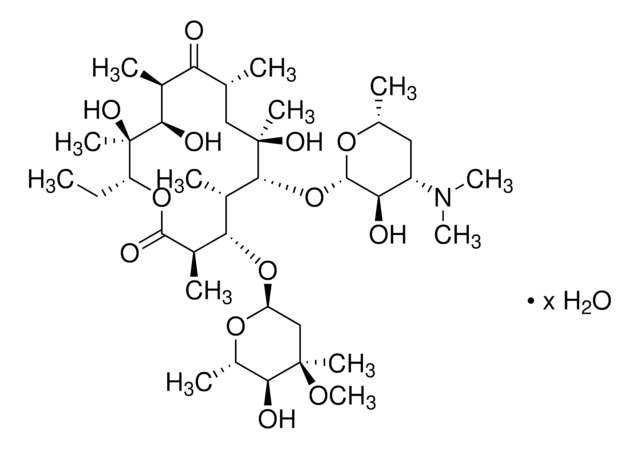
erythromycin
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
形式
neat
SMILES 字串
N(C1C(C(OC(C1)C)O[C@H]2[C@@](C[C@H](C(=O)[C@@H]([C@H]([C@H]([C@H](OC(=O)[C@@H]([C@H](C2C)OC3OC(C(C(C3)(OC)C)O)C)C)CC)C)O)C)C)(O)C)O)(C)C
InChI
1S/C37H67NO12/c1-14-26-20(4)29(40)21(5)28(39)18(2)16-36(9,44)33(50-35-30(41)25(38(11)12)15-19(3)46-35)22(6)31(23(7)34(43)48-26)49-27-17-37(10,45-13)32(42)24(8)47-27/h18-27,29-33,35,40-42,44H,14-17H2,1-13H3/t18-,19?,20+,21+,22?,23-,24?,25?,26-,27?,29+,30?,31+,32?,33-,35?,36-,37?/m1/s1
InChI 密鑰
IDRYSCOQVVUBIJ-ZTOAHLHUSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Erythromycin B EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
相關產品
產品號碼
描述
訂價
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
S Morimoto et al.
The Journal of antibiotics, 43(5), 544-549 (1990-05-01)
6-O-Methylerythromycin B has been synthesized from erythromycin B via regioselective methylation of the 6-hydroxyl group in 71% overall yield. This compound shows in vitro antibacterial activity comparable to erythromycins A and B and exhibits superior in vivo activity with improved
M S Brown et al.
The Journal of antibiotics, 52(8), 742-747 (1999-12-02)
Cyclopropane carboxylic acid was fed to Saccharopolyspora erythraea NRRL 18643 (6-deoxyerythromycin producer), resulting in the production of 6-deoxy-13-cyclopropyl-erythromycin B. These studies provide further evidence that deoxyerythronolide B synthase has a relaxed specificity for the starter unit.
Q Zhang et al.
Letters in applied microbiology, 52(2), 129-137 (2010-12-24)
To overproduce erythromycin C, B or D and evaluate the effect of disruption of tailoring genes eryK and eryG in an industrial erythromycin producer. The tailoring genes eryG and eryK were inactivated individually or simultaneously by targeted gene disruption in
M N Mordi et al.
Journal of medicinal chemistry, 43(3), 467-474 (2000-02-12)
One of the major drawbacks in the use of the antibiotic erythromycin A is its extreme acid sensitivity, leading to degradation in the stomach following oral administration. The modern derivative clarithromycin degrades by a different mechanism and much more slowly.
Sara Bogialli et al.
Rapid communications in mass spectrometry : RCM, 21(2), 237-246 (2006-12-16)
A rapid and simple sample preparation procedure for determining residues of antibiotics of the class of macrolides and lincomycin in whole milk and yoghurt by liquid chromatography/tandem mass spectrometry (LC/MS/MS) is presented. The method is based on the matrix solid-phase
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務