推薦產品
等級
analytical standard
品質等級
藥物控制
USDEA Schedule I; Home Office Schedule 1; regulated under CDSA - not available from Sigma-Aldrich Canada
技術
HPLC: suitable
gas chromatography (GC): suitable
應用
forensics and toxicology
pharmaceutical (small molecule)
veterinary
形式
neat
儲存溫度
2-8°C
SMILES 字串
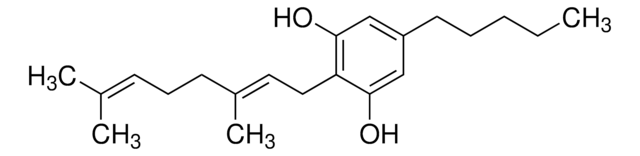
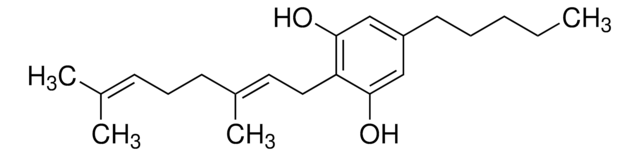
CCCCCc1cc(O)c-2c(OC(C)(C)c3ccc(C)cc-23)c1
InChI
1S/C21H26O2/c1-5-6-7-8-15-12-18(22)20-16-11-14(2)9-10-17(16)21(3,4)23-19(20)13-15/h9-13,22H,5-8H2,1-4H3
InChI 密鑰
VBGLYOIFKLUMQG-UHFFFAOYSA-N
基因資訊
human ... CNR2(1269)
rat ... Cnr1(25248)
尋找類似的產品? 前往 產品比較指南
應用
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
生化/生理作用
不作用于精神的四氢大麻酚的主要代谢物,具有免疫抑制特性的 CB2 大麻素受体激动剂。
其他說明
来自 C. sativa 的大麻组分
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral - Repr. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
客戶也查看了
A A Izzo et al.
Naunyn-Schmiedeberg's archives of pharmacology, 360(2), 221-223 (1999-09-24)
We have studied the effect of WIN 55,212-2 (a psychoactive cannabinoid agonist), cannabinol (a nonpsychoactive cannabinoid agonist), SR141716A, a cannabinoid CB1 antagonist, and SR144528, a cannabinoid CB2 antagonist, on gastric emptying in the rat. WIN 55,212-2 (0.1-5 mg/kg, i.p.) and
A C Herring et al.
The Journal of pharmacology and experimental therapeutics, 291(3), 1156-1163 (1999-11-24)
Cannabinol (CBN), an immunosuppressive cannabinoid and ligand for the peripheral cannabinoid receptor CB2, inhibits the cAMP signaling cascade in forskolin-stimulated thymocytes. The objective of the present studies was to further characterize the mechanism of CBN immune modulation by investigating its
S J MacLennan et al.
British journal of pharmacology, 124(4), 619-622 (1998-08-05)
The cannabinoid receptor antagonist SR141716A has been suggested to be an inverse agonist at CB1 receptors in some isolated intact tissues. We found that the basal incorporation of [35S]-GTPgammaS in Chinese hamster ovary cells expressing human recombinant CB1 and CB2
A C Herring et al.
Biochemical pharmacology, 55(7), 1013-1023 (1998-05-30)
Immune suppression by cannabinoids has been widely demonstrated in a variety of experimental models. The identification of two major types of G-protein-coupled cannabinoid receptors expressed on leukocytes, CB1 and CB2, has provided a putative mechanism of action for immune modulation
Penchal Reddy Nandaluru et al.
Organic letters, 14(1), 310-313 (2011-12-17)
A multicomponent domino reaction that affords 6H-dibenzo[b,d]pyran-6-ones is reported. The overall transformation consists of six reactions: Knoevenagel condensation, transesterification, enamine formation, an inverse electron demand Diels-Alder (IEDDA) reaction, 1,2-elimination, and transfer hydrogenation. Both the diene and dienophile for the key
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務