C2800000
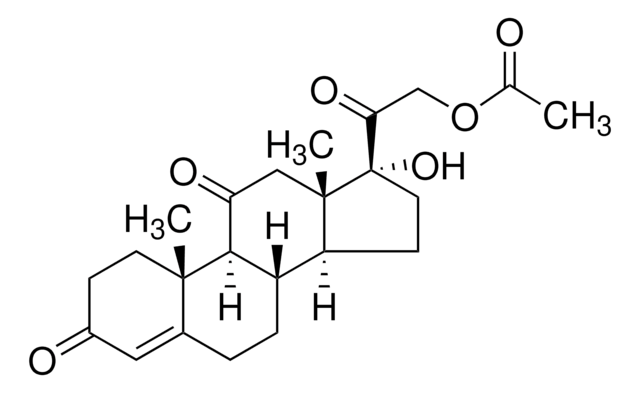
Cortisone acetate
European Pharmacopoeia (EP) Reference Standard
同義詞:
Cortisone 21-acetate, 17α,21-Dihydroxy-4-pregnene-3,11,20-trione 21-acetate, 21-Acetoxy-4-pregnen-17α-ol-3,11,20-trione, 4-Pregnene-17α,21-diol-3,11,20-trione 21-acetate
登入查看組織和合約定價
全部照片(1)
About This Item
經驗公式(希爾表示法):
C23H30O6
CAS號碼:
分子量::
402.48
Beilstein:
2067543
MDL號碼:
分類程式碼代碼:
41116107
PubChem物質ID:
NACRES:
NA.24
推薦產品
等級
pharmaceutical primary standard
API 家族
cortisone
製造商/商標名
EDQM
mp
237-240 °C (lit.)
應用
pharmaceutical (small molecule)
形式
neat
SMILES 字串
[H][C@@]12CCC3=CC(=O)CC[C@]3(C)[C@@]1([H])C(=O)C[C@@]4(C)[C@@]2([H])CC[C@]4(O)C(=O)COC(C)=O
InChI
1S/C23H30O6/c1-13(24)29-12-19(27)23(28)9-7-17-16-5-4-14-10-15(25)6-8-21(14,2)20(16)18(26)11-22(17,23)3/h10,16-17,20,28H,4-9,11-12H2,1-3H3/t16-,17-,20+,21-,22-,23-/m0/s1
InChI 密鑰
ITRJWOMZKQRYTA-RFZYENFJSA-N
基因資訊
human ... NR3C1(2908)
尋找類似的產品? 前往 產品比較指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Cortisone acetate EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
訊號詞
Warning
危險聲明
危險分類
Repr. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
L Barbetta et al.
Journal of endocrinological investigation, 28(7), 632-637 (2005-10-13)
Since the optimal glucocorticoid replacement needs to avoid over and under treatment, the adequacy of different daily cortisone acetate (CA) doses was assessed in 34 patients with primary and central hypoadrenalism. The conventional twice CA 37.5 mg/day dose was administered
Ricardo P P Moreira et al.
Clinics (Sao Paulo, Brazil), 66(8), 1361-1366 (2011-09-15)
21-hydroxylase deficiency is an autosomal recessive disorder that causes glucocorticoid deficiency and increased androgen production. Treatment is based on glucocorticoid replacement; however, interindividual variability in the glucocorticoid dose required to achieve adequate hormonal control has been observed. The present study
B Ekman et al.
Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme, 42(13), 961-966 (2010-10-07)
Our aim was to investigate the usefulness of circulating levels of adrenocorticotropic hormone (ACTH) and also salivary cortisol to monitor cortisone substitution in patients with Addison's disease. 13 patients with primary adrenal insufficiency (8 women and 5 men, age 44
K Løvås et al.
Journal of endocrinological investigation, 29(8), 727-731 (2006-10-13)
No ideal parameter is available for assessment of the glucocorticoid replacement therapy in Addison's disease. Serum cortisol day-curves can be used to monitor the therapy, but this technique is cumbersome and expensive. We evaluated the potential for saliva cortisol measurement
C Kristo et al.
Journal of endocrinological investigation, 31(5), 400-405 (2008-06-19)
Cardiovascular disease has been reported to be more common in patients with endogenous Cushing's syndrome (CS) compared to the normal population. In addition to altered lipid profile, inflammation seems to play an important pathogenic role in atherogenesis, but the role
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務



![双[4-(氧化缩水甘油)苯基]甲烷 mixture of isomers](/deepweb/assets/sigmaaldrich/product/structures/915/436/edb8eab6-834e-4238-a904-bf72c573b686/640/edb8eab6-834e-4238-a904-bf72c573b686.png)